| Identification | More | [Name]
Megestrol | [CAS]
3562-63-8 | [Synonyms]
17-ALPHA-HYDROXY-6-METHYL-4,6-PREGNADIENE-3,20-DIONE
4,6-PREGNADIEN-6-METHYL-17-OL-3,20-DIONE
MEGESTROL
17-hydroxy-6-methylpregna-4,6-diene-3,20-dione
20-dione,17-hydroxy-6-methyl-pregna-6-diene-3
17-alpha-hydroxy-6-methylpregna-4,6-diene-3,20-dione
Megestrol (base and/or unspecified salts) | [EINECS(EC#)]
222-628-6 | [Molecular Formula]
C22H30O3 | [MDL Number]
MFCD01939877 | [Molecular Weight]
342.47 | [MOL File]
3562-63-8.mol |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Uses]
Megestrol is an orally active progestogen. Megestrol is used in combinations as oral contraceptive and as antineoplastic agent. | [Definition]
ChEBI: A 3-oxo Delta4-steroid that is pregna-4,6-diene-3,20-dione substituted by a hydroxy group at position 17. | [Indications]
As a representitive of the compounds of the class of progestins, this drug is used for various
forms of cancer, in particular cancer of the breast, kidneys, and others. | [Brand name]
Megace (Bristol-Myers Squibb); Megace (Par);Citestrol;Co-ervonum;Combiquens;Femagest;Kombiquens;Megecat;Megeron;Megestat;Menoquens;Neo-delpregnin;Niaestine;Niagestine;Novaquin;Novokvens;Novolina;Novoquens;Oracolnal;Ovaban;Ovarid;Pallace;Serial 28;Volidan;Volplan. | [World Health Organization (WHO)]
Megestrol acetate, a synthetic progestogen, was introduced in the
early 1960s as a component in oral contraceptive preparations. In 1967, as a result
of new regulations required by the United States Food and Drug Administration,
megestrol acetate was submitted to long-term toxicity studies and by the early
1970s it was shown to be associated with an increased incidence of mammary
tumours in beagle bitches which led to its withdrawal by several regulatory
authorities. Subsequently the validity of the beagle bitch model as a predictor of
carcinogenicity of steroid contraceptives has been contested by many national
regulatory authorities and megestrol remains available in some countries for
contraceptive purposes. In other countries its use is restricted to anticancer
treatment.
(Reference: (WHODI) WHO Drug Information, 1-3, 5-7, 1984) | [Synthesis]
Megestrol, 17|á-hydroxy-6|á-methylpregna-4,6-dien-3,20-dione acetate
(28.3.9), is a product of dehydrogenation medroxyprogesterone (28.3.7) with chloranil
(tetrachloro-p-benzoquinone) in the presence of p-toluenesulfonic acid, which results in
the formation of an additional double bond at position C6¨CC7, and subsequent acetylation
of the product (28.3.8) leads to the desired megestrol (28.3.9) by acetic anhydride in the
presence of p-toluenesulfonic acid.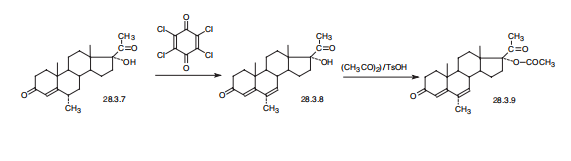
|
|
|