| Identification | More | [Name]
(BOC-AMINOOXY)ACETIC ACID | [CAS]
42989-85-5 | [Synonyms]
BOC-AOA
BOC-AOA-OH
BOC-AOAC-OH
BOC-(NHOAC)-OH
BOC-NH-O-CH2COOH
RARECHEM EM WB 0063
N-Boc-(carboxymethox
(BOC-AMINOOXY)ACETIC ACID
T-BOC-AMINOOXYACETIC ACID
BOC-3-(AMINOOXY)ACETIC ACID
tert-Boc-aminooxyaceticacid
(Tert-Butoxy)Carbonyl Aoa-OH
2-(BOC-AMINOOXY)-ACETIC ACID
N-BOC-(CARBOXYMETHOXY)-AMINE
N-BOC-(CARBOXYMETHOXY)-AMINO
(Boc-aMinooxy)acetic acid, 98+%
Boc-aminooxyacetic acid≥ 98% (HPLC)
(BOC-AMINOOXY)ACETIC ACID USP/EP/BP
BOC-AOA Tert-Boc-aMinooxyacetic acid
(Boc-aMinooxy)acetic acid >=98.0% (T)
Tert-boc-aminooxyacetic acid(Boc-AOA)
N-T-BUTOXYCARBONYL-(AMINOOXY)ACETIC ACID
N-tert-Butoxycarbonylaminooxyacetic acid
[(tert-Butoxycarbonyl)aminooxy]acetic Acid
2-(T-BUTYLOXYCARBONYL-AMINOOXY)-ACETIC ACID
2-((tert-Butoxycarbonyl)aMinooxy)acetic Acid
[(tert-Butoxycarbonyl)aminooxy]acetic Acid >
2-(TERT-BUTYLOXYCARBONYL-AMINOOXY)-ACETIC ACID
N-T-BUTOXYCARBONYL-HYDROXYLAMINE-O-ACETIC ACID
N-ALPHA-T-BUTOXYCARBONYL-(CARBOXYMETHOXY)-AMINE
[(tert-Butoxycarbonyl)aminooxy]acetic Acid
(Boc-aminooxy)acetic Acid
N-Boc-(carboxymethoxy)amine
2-[[[(1,1-DiMethylethoxy)carbonyl]aMino]oxy]acetic Acid
2-(Boc-aMinooxy)-acetic acid, N-Boc-(carboxyMethoxy)-aMine
Acetic acid, 2-[[[(1,1-dimethylethoxy)carbonyl]amino]oxy]-
Acetic acid, [[[(1,1-dimethylethoxy)carbonyl]amino]oxy]- (9CI)
(Boc-aminooxy)acetic acid, 98+% white powder | [Molecular Formula]
C7H13NO5 | [MDL Number]
MFCD01632027 | [Molecular Weight]
191.18 | [MOL File]
42989-85-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
~115 °C (dec.) | [density ]
1.214±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
Soluble in methanol. | [form ]
Solid | [pka]
2.97±0.10(Predicted) | [color ]
White to Off-White | [Usage]
A Boc-protected bifunctional linking reagent | [BRN ]
6137646 | [InChI]
InChI=1S/C7H13NO5/c1-7(2,3)13-6(11)8-12-4-5(9)10/h4H2,1-3H3,(H,8,11)(H,9,10) | [InChIKey]
QBXODCKYUZNZCY-UHFFFAOYSA-N | [SMILES]
C(O)(=O)CONC(OC(C)(C)C)=O | [CAS DataBase Reference]
42989-85-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [HazardClass ]
IRRITANT | [HS Code ]
29225090 |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
(Boc-aminooxy)acetic acid was employed to introduce a hydroxylamine moiety into peptides; reaction with an aldehyde to an oxime. It was used in the preparation of Boc-aminooxy tetra(ethylene glycol). | [Uses]
(Boc-aminooxy)acetic acid was used in the preparation of Boc-aminooxy tetra(ethylene glycol). | [Uses]
A Boc-protected bifunctional linking reagent | [reaction suitability]
reagent type: ligand | [Synthesis]
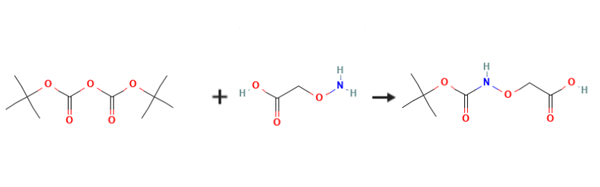
(Boc-aminooxy)acetic acid is prepared by the reaction of Di-tert-butyl dicarbonate and Aminooxyacetic acid. The specific synthesis steps are as follows:
A 500 mL round-bottom flask was charged with a suspension of aminooxyacetic acid (4 g, 36.6 mmol) in CH2Cl2 (100 mL).
The solution was cooled to 0° C. and triethylamine (16 mL, 114 mmol) was added dropwise while stiffing to dissolve all solids.
To this mixture was added a solution of di-tert-butyl dicarbonate (12 g, 55 mmol) in CH2Cl2 (20 mL).
The reaction was stirred for 30 min at 0° C., then continued for 1 h at rt.
The solution was washed with three 100 mL portions of water.
The aqueous portion was combined and extracted with two 100 mL portions of EtOAc; this EtOAc was discarded.
The pH of the aqueous portion was adjusted to 3.5 with 1 M HCl, then extracted with five 50 mL portions of EtOAc, adjusting to maintain pH 3.5 as needed.
The combined EtOAc was dried over Na2SO4, filtered, and the solvent removed in vacuo to afford a white crystalline powder (5.8 g, 83percent). TLC: (MeOH:CH2Cl2, 1:9) Rf=0.08. 1H NMR (400 MHz, CD3OD): δ 1.48 (s, 9H), 4.47 (s, 2H), 7.94 (s, 1H).
13C NMR (100 MHz, CD OD): δ, 27.1, 47.0, 72.3, 81.4, 157.8, 171.3.
 | [Solubility in organics]
Soluble in DCM, THF, DMF and DMSO |
|
|