| Identification | More | [Name]
CORTISONE | [CAS]
53-06-5 | [Synonyms]
11-DEHYDRO-17-HYDROXYCORTICOSTERONE
17A 21-DIHYDROXY-4-PREGNEN-3 17 20-TRIONE
17ALPHA,21-DIHYDROXY-4-PREGNENE-3,11,20-TRIONE
17ALPHA,21-DIHYDROXYPREGN-4-ENE-3,11,20-TRIONE
17ALPHA-HYDROXY-11-DEHYDROCORTICOSTERONE
17-HYDROXY-11-DEHYDROCORTICOSTERONE
4-PREGEN-17A,21-DIOL-3,11,20-TRIONE
4-PREGNEN-17,21-DIOL-3,11,20-TRIONE
4-PREGNEN-17ALPHA,21-DIOL-3,11,20-TRIONE
4-PREGNENE-17ALPHA,21-DIOL-3,11,20-TRIONE
CORTISONE
CORTONE
DELTA4-PREGNENE-17, 21-DIOL-3, 11, 20-TRIONE
DELTA4-PREGNENE-17ALPHA,21-DIOL-3,11,20-TRIONE
DELTA-PREGNENE-17ALPHA,21-DIOL-3,11,20-TRIONE
KENDALL'S CMPD ''E''
KENDALL'S COMPOUND E
KENDALL'S COMPOUND 'E'
KENDALL'S ''E''
REICHSTEIN'S COMPOUND ''FA'' | [EINECS(EC#)]
200-162-4 | [Molecular Formula]
C21H28O5 | [MDL Number]
MFCD00003610 | [Molecular Weight]
360.44 | [MOL File]
53-06-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Crystalline Powder | [Melting point ]
223-228 °C (dec.)(lit.)
| [alpha ]
D25 +209° (c = 1.2 in 95% alcohol); 25546 +269° (c = 0.125 in benzene); 25546 +248° (c = 0.1 to 0.2 in alcohol) | [Boiling point ]
412.46°C (rough estimate) | [density ]
1.28±0.1 g/cm3 (20 ºC 760 Torr) | [refractive index ]
210 ° (C=1, EtOH) | [Fp ]
9℃ | [storage temp. ]
-20°C Freezer | [solubility ]
Chloroform (Slightly, Heated, Sonicated), DMSO (Slightly), Ethanol (Slightly, Heated) | [form ]
Solid | [pka]
12.37±0.60(Predicted) | [color ]
White to Pale Beige | [Water Solubility ]
229.9mg/L(25 ºC) | [Usage]
Glucocorticoid, anti-inflammatory agent | [Merck ]
2539 | [LogP]
1.470 | [CAS DataBase Reference]
53-06-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Hazard]
Damaging side effects, e.g., sodium retention from ingestion. | [Chemical Properties]
Off-White Crystalline Powder | [Originator]
Cortone Acetate,MSD,US,1950 | [Uses]
Anticoagulant | [Uses]
antiinflammatory, glucocorticoid | [Uses]
Cortisone is used for inflammatory processes, allergies, and adrenal insufficiency. | [Uses]
Glucocorticoid, anti-inflammatory agent | [Definition]
ChEBI: A C21-steroid that is pregn-4-ene substituted by hydroxy groups at positions 17 and 21 and oxo group at positions 3, 11 and 20. | [Manufacturing Process]
The following technique is described in US Patent 2,541,104. A solution of 2.0
g of 3(α)-hydroxy-21-acetoxy-11,20-diketo-pregnane, which can be prepared
as described in Helv. Chim. Acta 27, 1287 (1944), is treated in a mixture of
25 cc of alcohol and 6.4 cc of acetic acid at 0°C with 6.0 g of potassium
cyanide. The solution is allowed to warm to room temperature and after 3
hours is diluted with water. The addition of a large volume of water to the
alcohol-hydrogen cyanide mixture precipitates a gum which is extracted with
chloroform or ethyl acetate. The extract is washed with water, and evaporated
to small volume under reduced pressure. The crystalline precipitate (1.3 g)
consists of 3(α),20-dihydroxy-20-cyano-21-acetoxy-11-keto-pregnane; dec.
175° to 185°C.
A solution of 0.60 g of chromic acid in 1.2 cc of water and 11 cc of acetic acid
is added to a solution containing about 1.2 g of 3(α),20-dihydroxy-20-cyano-
21-acetoxy-11-ketopregnane at room temperature. After 1 hour, water is
added and the product, which precipitates, is filtered and recrystallized from
ethyl acetate to produce 3,11-diketo-20-hydroxy-20-cyano-21-acetoxy�pregnane; dec. 214° to 217°C.
0.40 cc of phosphorus oxychloride is added to a solution containing about 950
mg of 3,11-diketo-20-hydroxy-20-cyano-21-acetoxy-pregnane dissolved in 3
cc of pyridine. After standing at room temperature for 24 hours, the solution
is poured into water and dilute hydrochloric acid, extracted with benzene and
concentrated to dryness. The crude product, after chromatography gives one
main constituent, namely δ17-3,11-diketo-20-cyano-21-acetoxy-pregnene; MP
189° to 190°C.
A solution of 1.0 g of δ17-3,11-diketo-20-cyano-21-acetoxy-pregnene in 10 cc
of benzene is treated with 1.0 g of osmium tetroxide and 0.43 g of pyridine.
After standing at room temperature for 18 hours, the resulting solution is
treated successively with 50 cc of alcohol, and with 50 cc of water containing
2.5 g of sodium sulfite. The mixture is stirred for 30 hours, filtered, and the
filtrate acidified with 0.5 cc of acetic acid and concentrated to small volume in
vacuo. The aqueous suspension is then extracted four times with chloroform,
the chloroform extracts are combined, washed with water and concentrated to
dryness in vacuo. Recrystallization of the residue from acetone gives 9°C. This
compound is then treated with acetic anhydride and pyridine for 15 minutes at
room temperature to produce 3,11,20-triketo-17(α)-hydroxy-21-acetoxy�pregnane or cortisone acetate. | [Therapeutic Function]
Glucocorticoid | [General Description]
Cortisone is a corticosteroid produced in the adrenal glands. Cortisone is administered for short term pain relief and to reduce swelling from inflammation. This Certified Spiking Solution? is applicable in LC-MS/MS applications for endocrinology, clinical chemistry and neonatal screening. | [Pharmacokinetics]
Following oral administration, cortisone acetate and hydrocortisone acetate are completely and
rapidly deacetylated by first-pass metabolism. Much of the oral cortisone, however, is inactivated by
oxidative metabolism before it can be converted to hydrocortisone in the liver. The pharmacokinetics
for hydrocortisone acetate is indistinguishable from that of orally administered hydrocortisone. Oral
hydrocortisone is completely absorbed, with a bioavailability of greater than 95% and a half-life of 1 to 2 hours
(23). | [Clinical Use]
Cortisone is administered orally or by intramuscular (IM) injection as its 21-acetate (cortisone acetate).Cortisone acetate or hydrocortisone usually is the corticosteroid of choice for replacement therapy in patients
with adrenocortical insufficiency, because these drugs have both glucocorticoid and mineralocorticoid
properties.
| [Synthesis]
Cortisone, 17|á,21-dihydroxypregn-4-en-3,11,20-trione (27.1.26), is also
synthesized in various ways from compounds already having the steroid skeleton. One of
them is very similar to a method of making hydrocortisone described above, in which it
is synthesized from progesterone, which undergoes microbiological oxidation, forming
11|á-hydroxyprogesterone (27.1.9). The hydroxyl group of the last is oxidized by
chromium(VI) oxide in acetic acid, giving 11-ketoprogesterone (27.1.10). This is reacted
with diethyloxalate in the presence of sodium ethoxide, forming the corresponding
|á-ketoester in the form of a sodium enolate 27.1.11, which undergoes bromination with
two equivalents of bromine, giving a dibromoketone 27.1.12. The resulting dibromoketone
undergoes a Favorskii rearrangement, but the product is not hydrolyzed, and the
unsaturated acid is isolated in the form of a methyl ester 27.1.20. Reacting this with
pyrrolidine gives a dienamine 27.1.21, which undergoes reduction by lithium aluminum
hydride, which results in that, the keto-group on C11 transforms into a hydroxyl group,
and the carbmethoxy group to a primary alcohol, forming the compound 27.1.22. Acidic
hydrolysis of the product and subsequent acetylation gives an acetate 27.1.23, and the
hydroxyl group at C11 in which it is oxidized with chromium(VI) oxide to a ketone, forming
the compound 27.1.24. This undergoes a reaction with osmium tetroxide, and the
resulting osmate is oxidized by magnesium dioxide in N-methylmorpholine, giving cortisone
acetate 27.1.25. Hydrolysis of the acetyl group using sodium bicarbonate leads to
the formation of cortisone (27.1.26).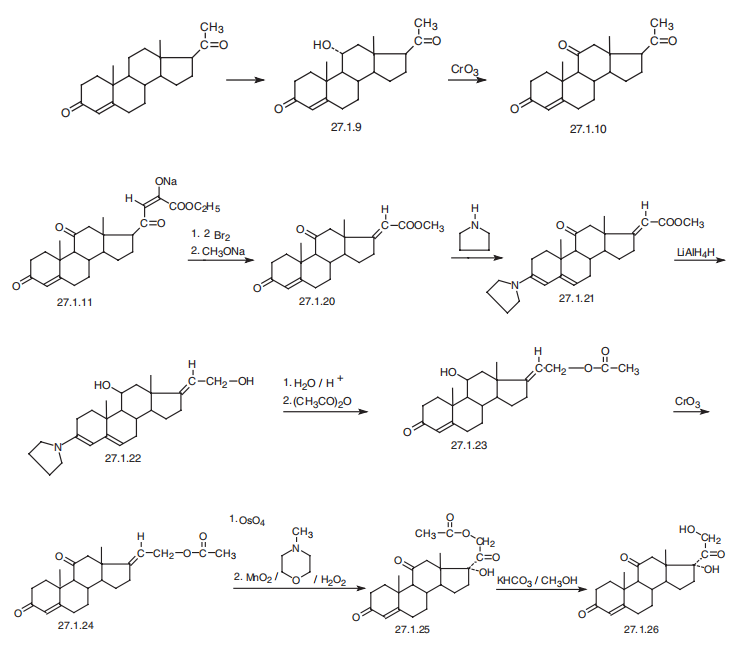
| [Metabolism]
The metabolism of hydrocortisone has been previously described. Cortisone acetate is slowly
absorbed from IM injection sites over a period of 24 to 48 hours and is reserved for patients who are unable to
take the drug orally. The acetate ester derivative demonstrates increased stability and has a longer duration of
action when administered by IM injection. Thus, smaller doses can be used. Similarly, hydrocortisone may be
dispensed as its 21-acetate (hydrocortisone acetate), which is superior to cortisone acetate when injected
intra-articularly. | [Purification Methods]
Crystallise cortisone from 95% EtOH or acetone. The UV has 14,000 M-1cm -1 at 237nm (EtOH). [Beilstein 8 IV 3480, Hems J Pharm Pharmacol 5 409 1953, Beilstein 8 IV 3480.] |
|
|