| Identification | More | [Name]
Iodomethyl pivalate | [CAS]
53064-79-2 | [Synonyms]
1-IODO-4-METHYLBENZENE
4-IODOTOLUENE
AURORA KA-7367
IODOMETHYL PIVALATE
IODOTOLUENE(4-)
P-IODOTOLUENE | [EINECS(EC#)]
210-841-7 | [Molecular Formula]
C6H11IO2 | [MDL Number]
MFCD00001059 | [Molecular Weight]
242.05 | [MOL File]
53064-79-2.mol |
| Chemical Properties | Back Directory | [Melting point ]
33-35 °C(lit.) | [Boiling point ]
211.5 °C(lit.) | [density ]
1.614±0.06 g/cm3(Predicted) | [refractive index ]
1.4830 to 1.4870 | [Fp ]
194 °F
| [storage temp. ]
Refrigerator, under inert atmosphere | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Oil | [color ]
Yellow | [Sensitive ]
Light, Air and Heat Sensitive | [InChI]
InChI=1S/C6H11IO2/c1-6(2,3)5(8)9-4-7/h4H2,1-3H3 | [InChIKey]
PELJISAVHGXLAL-UHFFFAOYSA-N | [SMILES]
C(OCI)(=O)C(C)(C)C | [CAS DataBase Reference]
53064-79-2(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Pale Yellow Liquid | [Uses]
Iodomethyl pivalate can be used as reagent used for the addition of pivaloyl group.
| [Synthesis Reference(s)]
The Journal of Organic Chemistry, 48, p. 5280, 1983 DOI: 10.1021/jo00174a024 | [Synthesis]
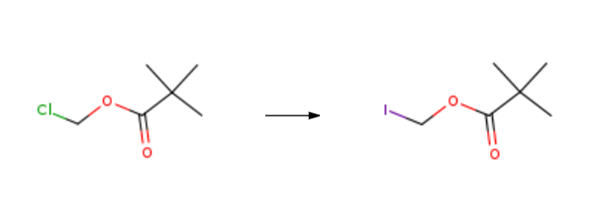
Iodomethyl pivalate is synthesised using chloromethyl pivalate as a raw material by chemical reaction. The specific synthesis steps are as follows:
Three bottles were added 10.0 g of chloromethyl pivalate, Ethyl acetate 30 mL, 11.6 g of sodium iodide and 3.6 g of calcium chloride, After heating to 78 ° C for 6 h, Cooling to 0 ,Then washed with 5% sodium thiosulfate to colorless, Dried over anhydrous magnesium sulfate, Concentrated under reduced pressure. 16 g of a yellow liquid. Iodomethyl pivalate was obtained. The reaction molar yield was 94% and GC: 98%.
 |
|
|