| Identification | Back Directory | [Name]
4-Iodo-2-(trifluoromethyl)pyridine | [CAS]
590371-73-6 | [Synonyms]
4-IODO-2-(TRIFLUOROMETHYL)PYRIDINE
2-(trifluoroMethyl)-4-iodopyridine
4-IODE-2-(TRIFLUOROMETHYL)PYRIDINE
Pyridine,4-iodo-2-(trifluoromethyl)-
4-Iodo-2-(trifluoromethyl)pyridine97%
4-Iodo-2-(trifluoromethyl)pyridine 97%
4-Iodo-2-(trifluoromethyl)pyridine ISO 9001:2015 REACH | [Molecular Formula]
C6H3F3IN | [MDL Number]
MFCD08234939 | [MOL File]
590371-73-6.mol | [Molecular Weight]
272.99 |
| Chemical Properties | Back Directory | [Melting point ]
24-26 °C | [Boiling point ]
206.2±35.0 °C(Predicted) | [density ]
1.974g/ml | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [form ]
liquid | [pka]
-0.82±0.20(Predicted) | [color ]
Clear, colourless to pale yellow |
| Hazard Information | Back Directory | [Uses]
4-Iodo-2-(trifluoromethyl)pyridine is used as a reagent in the synthesis of aminoisoindoles as β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitors which exhibit in vivo brain reduction of β-amyloid peptides. | [Reactivity Profile]
4-Iodo-2-(trifluoromethyl)pyridine could be cleanly deprotonated by
LIDA in tetrahydrofuran at –75 °C[1]. Trapping of the organometallic intermediates with dry ice followed by neutralization gave the 4-iodo-2-(trifluoromethyl)pyridine-3-carboxylic acid (16, 31%). a: LIDA in THF at –75 °C for 2 h; b: (1.) Excess solid
carbon dioxide, (2.) Hydrochloric acid.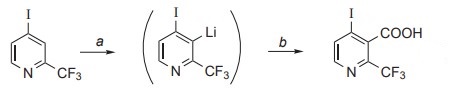 | [References]
[1] Schlosser M, et al. Further Metalations and Functionalizations of Chloro-, Bromo- and
Iodo(trifluoromethyl)pyridines. Synthesis, 2004; 10: 1619-1624. |
|
|