| Identification | More | [Name]
Phenethyl alcohol | [CAS]
60-12-8 | [Synonyms]
2-PHENYLETHANOL
2-PHENYLETHYL ALCOHOL
AKOS BBS-00004355
BENZENEETHANOL
BENZYL CARBINOL
BETA PHENYL ETHYL ALCOHOL
B-HYDROXYETHYLBENZENE
FEMA 2858
PEA
PHENETHYL ALCOHOL
PHENYL ETHANOL
PHENYLETHANOL,2-
PHENYL ETHYL ALCHOL
PHENYL ETHYL ALCOHOL
RARECHEM AL BD 0140
(beta-pea)
.beta.-Phenylethanol
1-Phenyl-2-ethanol
2-PEA
2-Phenethanol | [EINECS(EC#)]
200-456-2 | [Molecular Formula]
C8H10O | [MDL Number]
MFCD00002886 | [Molecular Weight]
122.16 | [MOL File]
60-12-8.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless liquid | [Melting point ]
-27 °C (lit.) | [Boiling point ]
219-221 °C/750 mmHg (lit.) | [density ]
1.020 g/mL at 20 °C(lit.)
| [vapor density ]
4.21 (vs air)
| [vapor pressure ]
1 mm Hg ( 58 °C)
| [FEMA ]
2858 | [refractive index ]
n20/D 1.5317(lit.)
| [Fp ]
216 °F
| [storage temp. ]
Store at RT. | [solubility ]
Miscible with chloroform. | [form ]
Liquid | [pka]
15.17±0.10(Predicted) | [color ]
Clear colorless | [Odor]
floral odor of roses | [PH]
6-7 (20g/l, H2O, 20℃) | [Stability:]
Stable. Substances to be avoided include strong acids and strong oxidizing agents. Combustible. | [biological source]
synthetic | [explosive limit]
1.4-11.9%(V) | [Odor Type]
floral | [Water Solubility ]
20 g/L (20 ºC) | [JECFA Number]
987 | [Merck ]
14,7224 | [BRN ]
1905732 | [Dielectric constant]
13.0(20℃) | [InChIKey]
WRMNZCZEMHIOCP-UHFFFAOYSA-N | [LogP]
1.50 | [Uses]
phenethyl alcohol is used to mask odor and also as a preservative. | [CAS DataBase Reference]
60-12-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Benzeneethanol(60-12-8) | [EPA Substance Registry System]
60-12-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R21/22:Harmful in contact with skin and if swallowed .
R36/38:Irritating to eyes and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37:Wear suitable protective clothing and gloves .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
2810 | [WGK Germany ]
1
| [RTECS ]
SG7175000
| [Autoignition Temperature]
410 °C | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29062990 | [Safety Profile]
Moderately toxic by ingestion and skin contact. A skin and eye irritant. Experimental teratogenic effects. Other experimental reproductive effects. Causes severe central nervous system injury to experimental animals. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidzing materials. To fight fEe, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes | [Hazardous Substances Data]
60-12-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: 1790 mg/kg (Jenner) |
| Questions And Answer | Back Directory | [Edible spices]
Phenethyl alcohol is a kind of edible spices, and naturally exists in neroli, rose oil, geranium oil and other oils, because it has a soft, pleasant and persistent rose fragrance and is widely used in various kinds of flavors and cigarette flavor. It is dispensing rose scent, food additives, the main raw material for rose scent flavor, stable on alkali, which are widely used in soap fragrance, is essence blending all rose scent series of spices, because it does not dissolve in water, it is often used in the making up water, soap and orange flower, purple, etc. It is also used in the blending of flavor. Because the Phenethyl alcohol has a good antibacterial efficiency, it can be used in the ophthalmic solution. At present there are main three synthesis methods as following:
1, by styrene via halogenation, saponification, hydrogenation, distillation.
2, and microorganism fermentation in yeast by bioconversion.
3, calcium carbide, benzene as raw material preparation of benzyl ethanol, reaction equations are as follows:
1)CaC2+2H2O=Ca(OH)2+C2H2
2)C6H6+C2H2=C6H6CHCH2(Styrene)
3)C6H6CHCH2+H2O=C6H6CH2CH2OH(Phenylethyl alcohol)
| [Chemical Properties]
Phenethyl alcohol is a clear, colorless liquid with an odor of rose oil. It has a burning taste that irritates and then anesthetizes mucous membranes.

Phenethyl Alcohol (PEA) is an aromatic alcohol that is used as a fragrance and an antimicrobial preservative in cosmetic formulations. It is active at pH 6 or less and is inactivated by nonionic detergents including polysorbate-80. PEA is also a widely used fragrance material that imparts a rose character to perfume compositions. Almost all rose fragrances and other floral-type perfumes contain PEA, and PEA is used extensively for many other fragrance applications because it blends ell.
PEA is metabolized to phenylacetic acid in mammals. In humans, it is excreted in urine as the conjugate phenylacetylglutamine.
| [Production]
Many syntheticmethods are known for preparing phenylethyl alcohol; the following are currently of industrial importance:
1) Friedel–Crafts reaction of benzene and ethylene oxide: In the presence of molar quantities of aluminum chloride, ethylene oxide reacts with benzene to give an addition product, which is hydrolyzed to phenylethyl alcohol:
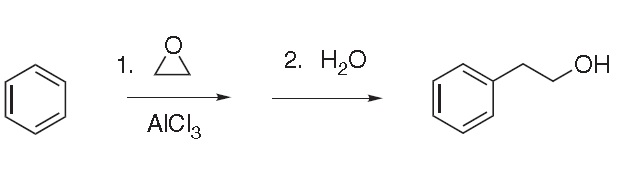
Formation of by-products, such as 1,2-diphenylethane, is largely avoided by using an excess of benzene at low temperature. Special purification procedures are required to obtain a pure product that is free of chlorine and suitable for use in perfumery.
2) Hydrogenation of styrene oxide: Excellent yields of phenylethyl alcohol are obtainedwhen styrene oxide is hydrogenated at low temperature, using Raney nickel as a catalyst and a small amount of sodium hydroxide.
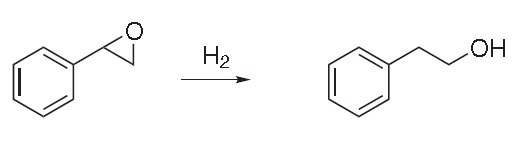
|
| Hazard Information | Back Directory | [Occurrence]
Reported found (as is or esterified) in several natural products: rose concentrate, rose absolute (60% or more)
and rose distillation waters; also found in the essential oils of neroli, ylang-ylang, narcissus, hyacinth, lily, tea leaves, Michelia champaca,
Pandamus odoratissimus, Congo and Réunion geranium, tobacco and other oils. It has been identified in wines. It has also been reported found in over 200 foods and beverages including apple, apricot, orange juice, orange peel, many berries, bilberry, cherry,
grapefruit, peach, raisin, blackberry, guava, grapes, melon, papaya, asparagus, cabbage, leek, potato, rutabaga, tomato, Mentha
oils, cinnamon, ginger, breads, butter, saffron, mustard, mango, many cheeses, butter, milk, cooked chicken, cognac, hop oil, beer,
rum, whiskies, cider, sherry, cocoa, coffee, tea, nuts, oats, honey, soybean, coconut meat, avocado, olive, passion fruit, plum, beans,
mushroom, starfruit, mango, tamarind, fruit brandies, fig, gin, rice, quince, radish, litchi, sukiyaki, calamus, licorice, buckwheat,
watercress, elderberry fruit, kiwifruit, loquat, Tahiti and Bourbon vanilla, mountain papaya, endive, lemon balm, clary sage, shrimps,
crab, Chinese quince, lamb’s lettuce, truffle and maté. | [Definition]
ChEBI: 2-phenylethanol is a primary alcohol that is ethanol substituted by a phenyl group at position 2. It has a role as a fragrance, a Saccharomyces cerevisiae metabolite, a plant metabolite, an Aspergillus metabolite and a plant growth retardant. It is a primary alcohol and a member of benzenes. | [Preparation]
From toluene, benzene or styrene. | [Production Methods]
Phenylethyl alcohol is prepared by reduction of ethyl phenylacetate
with sodium in absolute alcohol; by hydrogenation of phenylacetaldehyde
in the presence of a nickel catalyst; or by addition of
ethylene oxide or ethylene chlorohydrin to phenylmagnesium
bromide, followed by hydrolysis. Phenylethyl alcohol also occurs
naturally in a number of essential oils, especially rose oil. | [Aroma threshold values]
Detection: 0.015 ppb to 3.5 ppm; recognition: 1.2 ppm. Aroma characteristics at 1.0%: floral honey, yeasty
bready, musty fresh and sweet. | [Taste threshold values]
Taste characteristics at 20 ppm: mushroom-like, rose floral, sweet, rosy, bready with honey nuances. | [Synthesis Reference(s)]
Chemistry Letters, 18, p. 619, 1989
Journal of the American Chemical Society, 100, p. 4888, 1978 DOI: 10.1021/ja00483a042
Tetrahedron Letters, 18, p. 3263, 1977 DOI: 10.1016/S0040-4039(01)83213-5 | [General Description]
Phenylethyl alcohol, is a primary aromatic alcohol of high boiling point, having a characteristic rose-like odor. It presents organoleptic properties and impacts the quality of the wine, distilled beverages, and fermented foods. It shows its presence in fresh beer and is responsible for the rose-like odor of well-ripened cheese. It is commercially and industrially an important flavor and is a component of a variety of foodstuffs such as ice cream, gelatin, candy, pudding, chewing gum, and non-alcoholic beverages. It is formed by yeasts during fermentation of alcohols either by decomposition of L-phenylalanine or metabolism of sugar substrates.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. | [Health Hazard]
Phenylethanol is an irritant of
the eyes and a teratogen in rats. | [Pharmaceutical Applications]
Phenylethyl alcohol is used as an antimicrobial preservative in
nasal, ophthalmic, and otic formulations at 0.25–0.5% v/v
concentration; it is generally used in combination with other
preservatives.Phenylethyl alcohol has also been used on its own
as an antimicrobial preservative at concentrations up to 1% v/v in
topical preparations. At this concentration, mycoplasmas are
inactivated within 20 minutes, although enveloped viruses are
resistant.Phenylethyl alcohol is also used in flavors and as a
perfumery component, especially in rose perfumes. | [Safety]
Phenylethyl alcohol is generally regarded as a nontoxic and
nonirritant material. However, at the concentration used to preserve
eye-drops (about 0.5% v/v) or above, eye irritation may occur.
LD50 (rabbit, skin): 0.79 g/kg
LD50 (rat, oral): 1.79 g/kg | [Carcinogenicity]
Phenylethanol was not mutagenic in
bacterial assays, nor did it increase the number
of sister chromatid exchanges in human
lymphocytes. | [Metabolism]
Phenylethyl alcohol is oxidized almost entirely to the corresponding acid (Williams. 1959). | [storage]
Phenylethyl alcohol is stable in bulk, but is volatile and sensitive to
light and oxidizing agents. It is reasonably stable in both acidic and
alkaline solutions. Aqueous solutions may be sterilized by
autoclaving. If stored in low-density polyethylene containers,
phenylethyl alcohol may be absorbed by the containers. Losses to
polypropylene containers have been reported to be insignificant
over 12 weeks at 30°C. Sorption to rubber closures is generally
small.
The bulk material should be stored in a well-closed container,
protected from light, in a cool, dry place. | [Purification Methods]
Purify the ethanol by shaking it with a solution of ferrous sulfate, and the alcohol layer is washed with distilled water and fractionally distilled. [Beilstein 6 IV 3067.] | [Incompatibilities]
Incompatible with oxidizing agents and protein, e.g. serum.
Phenylethyl alcohol is partially inactivated by polysorbates,
although this is not as great as the reduction in antimicrobial
activity that occurs with parabens and polysorbates. | [Regulatory Status]
Included in the FDA Inactive Ingredients Database (nasal,
ophthalmic, and otic preparations). Included in nonparenteral
medicines licensed in the UK. Included in the Canadian List of
Acceptable Non-medicinal Ingredients. |
|
|