| Identification | More | [Name]
Sodium [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]methanesulfonate | [CAS]
68-89-3 | [Synonyms]
ANALGIN
BENZALAGEN
dipyron
DIPYRONE
DIPYRONE SODIUM
LABOTEST-BB LT00771955
METAMIZOLE
metamizole sodium
METAMIZOL SODIUM
SODIUM [(1,5-DIMETHYL-3-OXO-2-PHENYL-PYRAZOL-4-YL)-METHYL-AMINO]METHANESULFONATE
sodium [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1h-pyrazol-4-yl)methylamino]methanesulfonate
SODIUM DIPYRONE
sulpyrine
(antipyrinylmethylamino)methanesulfonicacidsodiumsalt
(antipyrinylmethylamino)-methanesulfonicacimonosodiumsalt
(antipyrinylmethylamino)-methanesulfonicacisodiumsalt
1-phenyl-2,3-dimethyl-4-methylamino-5-pyrazolon-n-methanesulfonsaeurennatriu
1-phenyl-2,3-dimethyl-5-pyrazolone-4-methylaminomethanesulfonatesodium
1-phenyl-2,3-dimethylpyrazolone-(5)-4-methylaminomethanesulfonicacidsodium
4-methylamino-1,5-dimethyl-2-phenyl-3-pyrazolonesodiummethanesulfonate | [EINECS(EC#)]
200-694-7 | [Molecular Formula]
C13H16N3NaO4S | [MDL Number]
MFCD00020783 | [Molecular Weight]
333.34 | [MOL File]
68-89-3.mol |
| Chemical Properties | Back Directory | [Appearance]
solid | [Melting point ]
227 °C | [density ]
1.388 g/cm3 | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), Methanol (Slightly), Water (Slightly) | [form ]
Solid | [color ]
Minute crystals | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [InChI]
InChI=1S/C13H17N3O4S.Na/c1-10-12(14(2)9-21(18,19)20)13(17)16(15(10)3)11-7-5-4-6-8-11;/h4-8H,9H2,1-3H3,(H,18,19,20);/q;+1/p-1 | [InChIKey]
DJGAAPFSPWAYTJ-UHFFFAOYSA-M | [SMILES]
[Na+].C(N(C1=C(C)N(C)N(C2=CC=CC=C2)C1=O)C)S([O-])(=O)=O | [CAS DataBase Reference]
68-89-3(CAS DataBase Reference) | [NIST Chemistry Reference]
Dipyrone(68-89-3) | [EPA Substance Registry System]
Methanesulfonic acid, 1-[(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]-, sodium salt (1:1) (68-89-3) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S36:Wear suitable protective clothing . | [WGK Germany ]
2
| [RTECS ]
PB1300000
| [HS Code ]
29331920 | [Safety Profile]
Poison by subcutaneous route. Moderately toxic by several other routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. Questionable carcinogen with experimental neoplastigenic data. See also SULFONATES. When heated to decomposition it emits very toxic fumes of NOx,, Na2O, and SOx,. | [Toxicity]
LD50 orl-rat: 3 g/kg ARZNAD 21,719,71 |
| Questions And Answer | Back Directory | [Description]
Dipyrone is a water soluble pyrazolone derivative available in oral,
rectal and injectable forms. It has analgesic, antipyretic,
antispasmodic, and anti-inflammatory properties.
Dipyrone is used most commonly to treat severe pain, particularly,
for pain associated with smooth muscle spasm or colic affecting the
gastrointestinal, biliary or urinary tracts. It is useful for fever
that is refractory to other treatment. Because of the risk of
serious adverse effects, such as agranulocytosis, its use is
justified only in serious situations where no alternative is
available or suitable. | [Drug Instructions]
Alias: dipyrone, NOVALGIN; Noramidopyrine, Metamizole sodium,metamizole
Property: This product is white or yellow-white crystal or crystalline powder, odorless, slightly bitter taste, soluble in water.
Action: Dipyrone is the derivative of aminopyrine, its has a significant effect of antipyretic analgesia. The antipyretic effect of it is three times as aminopyrine, the analgesic effect is similar to that of aminopyrine.
Process vivo: Easily absorbed orally, mainly metabolized by the liver and excreted of renal
Indications: It is mainly used for cooling, acute arthritis, headache, rheumatic neuralgia, toothache and muscle pain etc.
Dosage
Oral or intranasal administration. For oral, the adult should take 0.5g of the medicine and three times a day, the amount of the child is 8-10mg/ kg, the number of the times is according to the necessary. For intranasal, the child under 5 years old can take 1-2drops for each nostril, can take the medicine another time if necessary,for the patient above 5 years old increase the dosage appropriately.
Note
1, The dosage should be controled strictly to prevent collapse.
2, The intramuscular injection can cause atrophy and erosion to the local muscle, it is abandoned now.
3, The patient who has a allergy to the praziquantel can not use the dipyrone
4, It not suitable for a long-term application, Please pay attention to the granulocytes.
Specification
Tablets:each table is weight for 0.25g or 0.5g; Injection: each ingection has the specification of 0.25g/1ml or 0.5g/2ml; Drops: solution with the concentration of 10%~20% | [Side effects of dipyrone]
1, The side effects contain the allergies, exhaustion, leukopenia, thrombocytopenia even the severe aplastic anemia.
2, Some people may also suffering the nephrotoxicity and gastrointestinal bleeding after they take the dipyrone.The most serious side effect is lethal agranulocytosis. | [Uses]
It has the effect of detoxification, analgesic and anti-rheumatic etc and mainly used for cooling, but also can be applied to cure the acute arthritis, rheumatic pain, muscle pain and headache etc. | [Hazards & Safety Information]
Category
Toxic Chemicals
Acute toxicity
For oral – the lethal dose for rat LD50: 3000 mg/kg;
For oral – the lethal dose for mouse LD50: 2891 mg/kg
Toxicity grading
Moderately toxic
Flammability hazard characteristic
It is combustible and can produce the toxic fumes contained nitrogen oxides, sulfur oxides and sodium oxide during the combustion
Storage Characteristic
The storeroom should be airy, low-temperature and dried.
Extinguishing agent
Dry powder, foam, sand, carbon dioxide, water mist | [References]
[1] Micha Levy, Ester Zylber-Katz and Bernd Rosenkranz, Clinical Pharmacokinetics of Dipyrone and its Metabolites, Clinical Pharmacokinetics, 1995, vol. 28, 216-234
[2] JE Edwards, F. Meseguer, CC Faura, RA Moore and HJ McQuay, Single-dose dipyrone for acute postoperative pain, Cochrane Database Syst Rev., 2010, vol. 9, CD003227
|
| Hazard Information | Back Directory | [Chemical Properties]
solid | [Definition]
ChEBI: An organic sodium salt of antipyrine substituted at C-4 by a methyl(sulfonatomethyl)amino group, commonly used as a powerful analgesic and antipyretic. | [Brand name]
Diprofarn (Farmitalia, Societa Farmaceutici Italia, Italy); Novaldin (Sterling Winthrop);Abalgine;Acabel compositum;Acefalgin;Acrobal;Acrogesico;Adolkin;Algia-nil;Alginodia compose;Algisedal;Algobuscopan;Algopriv;Algopyriv;Alkozin;Amiglan;Aminocid;Amitralil;Ampi tumisan;Anadex;Analcedor;Analject;Anarinyl;Anchrina;Anespas cpto;Angiter;Ankaljin;An-t;Apasmo;Arantil;Arquidon;Artritex;Ascorbalgine;Ascortin;Aseptobron;Atecilina;Atn-020/2;Avafortan;Ayoral;Bayer 1387;Bebealjin;Bebigut;Belatropin;Belflex/2;Beneurin;Bexopirona;Biogamma2;Biotangin;Bipasmin compuesto;Bort;Bristacilia;Britercina;Bromalgin;Bromalgon;Broncofenil;Broncolysin;Bucarboxal;Buscapina comp.;Buscapina compuesto;Buscapina compuestum;Buscol compositum;Buscopan composto;Buscopan compostum;Buscopina compostum;Butalgine;Butylpan;Calgayan-c;Calmetron;Camizol;Causalon;Cessantyl;Chini-med;Cintaverin compuesto;Clizim;Clofexan;Codalgin;Codasal injetavel;Colgenol;Comaril 5000;Corilin pediatric;Cortempirol;Cortitracin;Cronopen balsamico;Deltricin;Devalgin;Dexa butarin;Di-bal-rone;Dimethedon;Dinopirina;Dioxadol;Dipirona;Diprofarm;Dipyrivo;Dispalgine;Divarin;Divarmin;Do-ba-rone;Dolatets;Dolazon;Dolemicin;Dolispasmo;Dolo adamon;Dolo baralgine;Dolo buscopan;Dolo nerv;Dolo neurobion forte;Dolo pangavit;Dolo raptalgin;Dolo spasuret;Dolojudolor;Dolo-neurobion;Dolopirina;Doloscopin;Dopiral;Dorflex merrell;Dorlisin;Doron;Dorscopena;D-pron;Dumalgin;Duralnordin;Dya-tran;Edgartet;Eespanal;Enzipan combinado;Espasfher;Espasmir;Espasmo-cibalgina;Espasmoqual;Espasmotex;Espasmoviral;Espyre;Farbinol;Flogolisin;G.r. ulix compuesto;Genservet;Gentil;Geralgine;Glutisal;Greplicina belsa;H 116;H 117;H 118;Hagalgin;Hasain;Indextron;Influbene;Kb-502;Kefren;Kesan;Killgrip;Kipyrone;Kitax alpha;Kitax n;Konitan;Labymetacincpo;Lactmicina;Lagalgine;Lamprcsnum;Lapalgine;Larq 731;Lasain;Lavaciclina;Levapa;Levismon;Lisalgil;Magdor;Magnalsa;Magnemidon;Mapir;Mecoten;Megal;Melpen;Menalgine;Mialgan;Minalgine;Minoval;Miocitalgan;Nadalgine;Naftalgin;Naltrium;Napasone;Nartate;Natralgin;Natric;Neo-melubrim;Neo-melubrina;Neo-melubrine;Neo-oxipen;Neosal-n;Neosoldina;Neuro-fortamin;Nisidina;Nlo conicilina balsamica;Nobelgin;Nolotil composirum;Notermin;Novacid;Novalcina;Novalgetol;Novalgin quinine;Novalgina;Novalgine;Nova-lyseen;Novamidazofen;Novamidazophen;Novamideazophene;Novaminophenazone;Novaminsulfon ratiopharm;Novaminsulfone sodium;Novaminsulfonium;Novaminsulfonum;Novaminsulton;Novazolon dexametasona;Noveltex;Novemida;Novemina;Novil;Oftlamin;Orphalginen;Ortopirona;Oxiquiunazine;Pabron gold;Panalvon;Patalgin;Pentrodin;Phanalgin;Pharmalgine;Porbiot;Pplan 2500;Probaphen;Prodol;Prydonnal;Pydirone;Pyralgine;Pyrilgin;Pyriligin;Pyrisan;Quarelin;Reflex rectal;Relexal compuesto;Repriman;Resquim;Rheuma-spalt;Ridol;Rumalisine;Rupalgin;Santeprednisan a;Sebon;Sedabel;Sedarel;Sedarene;Sedazepane;Selpiran;Sertalanalgesico;Severen;Sinalgex;Sintaverin;Sinvirol;Spasdolsom;Spaslar;Spasmalgon;Spasmium-comp.;Spasmizol;Spasmodor;Spasmopyralgin;Spasmothil;Sufonovin;Sulfonovin;Supadol;Supergine;Surpyrine;Tanper;Tapal;Tega-pyrone;Tempil;Tepal;Termonil;Tetrabal-hosbon;Tetraspasmil;Tiadexol;Tiartan;Toloxin andromaco;Trenteron;Triartan;Trinalgen. | [World Health Organization (WHO)]
Metamizole sodium, a pyrazolone derivative with analgesic,
antipyretic and antinflammatory activity, was introduced in 1921 and has since been
widely available in over-the-counter products. By the early 1970s its use had been
associated, as with some other pyrazolones, with serious and sometimes fatal adverse
reactions, notably cases of blood dyscrasias including agranulocytosis, which led to
its withdrawal by some regulatory authorities. Although preparations of
metamirole sodium are prohibited in certain countries, they remain widely available in
others and, in some cases, in over-the-counter products. | [Synthesis]
Methamizole sodium, 1-phenyl-2,3-dimethyl-4-methylaminopyra�zolone-5-N-sodium methansulfonate (3.2.16), is synthesized in a multi-stage synthesis from
acetoacetic ester and phenylhydrazine. Their reaction leads to the formation of 1-phenyl-
3-methylpyrazolone-5 (3.2.9). Methylation of this product with methyl iodide gives 1-phenyl-
2,3-dimethylpyrazolone-5 (3.2.10). This compound is used independently in medicine as a
fever-reducing and anti-inflammatory analgesic under the name antipyrin. It undergoes
nitrozation by sodium nitrite in an acidic medium, forming 1-phenyl-2,3-dimethyl-4-
nitrozopyrazolone-5 (3.2.11). Reduction of the nitrous derivative (3.2.11) by different reduc�ing agents leads to the formation of 1-phenyl-2,3-dimethyl-4-aminopyrazolone-5 (3.2.12).
This product is reacted with benzaldehyde, forming an easily separable crystalline 1-phenyl-
2,3-dimethyl-4-benzylidenaminopyrazolone-5 (3.2.13), which is methylated at the imine atom
of nitrogen by dimethylsulfate, giving a quaternary salt (3.2.14). Hydrolysis of the resulting
salt gives 1-phenyl-2,3-dimethyl-4-methylaminopyrazolone-5 (3.2.15). Treating the product
with a water solution of a mixture of sodium bisulfite and formaldehyde leads to the forma�tion of 1-phenyl-2,3-dimethyl-4-methylaminopyrazolone-5-N-sodium methanesulfonate
(3.2.16), the desired sodium methamizole [72¨C75].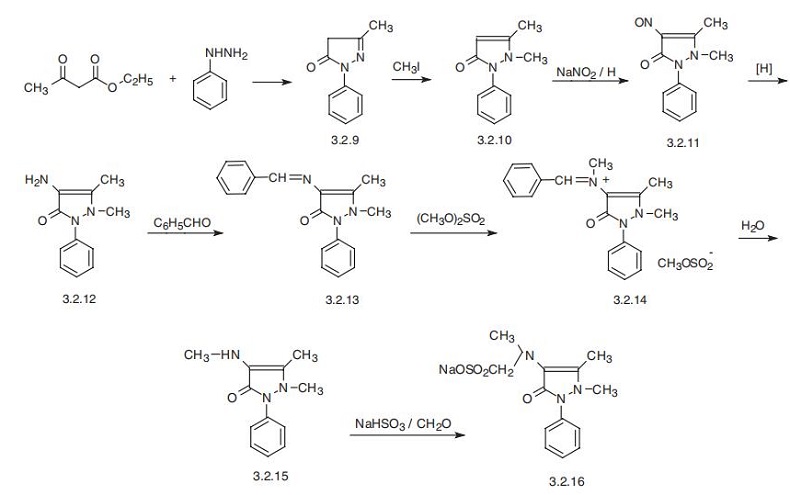
|
|
|