| Identification | More | [Name]
Lithium amide | [CAS]
7782-89-0 | [Synonyms]
LITHIUM AMIDE
lithamide
Lithiumamideoffwhitepowder
LITHIUM AMIDE, HYDROGEN STORAGE GRADE
LITHIUM AMIDE, POWDER, 95%
Lithiumamide,95%
Lithium amide (Li(NH2))
Lithiumamid
lithium azanide
Aminolithium
Lithioamine
Litium amide | [EINECS(EC#)]
231-968-4 | [Molecular Formula]
H2LiN | [MDL Number]
MFCD00011093 | [Molecular Weight]
22.96 | [MOL File]
7782-89-0.mol |
| Chemical Properties | Back Directory | [Appearance]
white to grey fine powder | [Melting point ]
373 °C | [Boiling point ]
430 °C | [density ]
1.178 g/mL at 25 °C(lit.)
| [refractive index ]
1.178g/mL | [storage temp. ]
water-free area | [solubility ]
Slightly soluble in ethanol and liquid ammonia. Insoluble in anhydrous ether, benzene and toluene. | [form ]
powder
| [color ]
off-white | [Specific Gravity]
1.178 | [Water Solubility ]
reacts | [Sensitive ]
Air & Moisture Sensitive | [Merck ]
14,5522 | [InChIKey]
AFRJJFRNGGLMDW-UHFFFAOYSA-N | [CAS DataBase Reference]
7782-89-0(CAS DataBase Reference) | [EPA Substance Registry System]
7782-89-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,C | [Risk Statements ]
R14/15:Reacts violently with water, liberating extremely flammable gases .
R29:Contact with water liberates toxic gas.
R34:Causes burns.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R15:Contact with water liberates extremely flammable gases.
R14:Reacts violently with water.
R11:Highly Flammable. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add-Never use water) .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S7/8:Keep container tightly closed and dry .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 1390 4.3/PG 2
| [WGK Germany ]
2
| [RTECS ]
OJ5590000 | [TSCA ]
Yes | [HazardClass ]
4.3 | [PackingGroup ]
II | [HS Code ]
28530090 | [Safety Profile]
A powerful irritant to
skin, eyes, and mucous membranes.
Flammable when exposed to heat or flame.
Ammonia is liberated and lithmm hydroxide
is formed when this compound is exposed
to moisture. Reacts violently with water or
steam to produce toxic and flammable
vapors. Vigorous reaction with oxilzing
materials. Exothermic reaction with acid or
acid fumes. When heated to decomposition
it emits very toxic fumes of LiO, NH3, and
NO,. Used in synthesis of drugs, vitamins,
steroids, and other organics. See also
LITHIUM COMPOUNDS, AMIDES,
AMMONIA, and LITHIUM
HYDROXIDE. | [Hazardous Substances Data]
7782-89-0(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
White crystalline powder with an odor of ammonia. Denser than water. | [Reactivity Profile]
Powdered LITHIUM AMIDE is highly reactive. A strong base. Reacts to release toxic ammonia gas with water. Forms explosive peroxide on storage. | [Health Hazard]
Highly toxic: contact with water produces toxic gas, may be fatal if inhaled. Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. | [Fire Hazard]
Produce flammable and toxic gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Containers may explode when heated. Runoff may create fire or explosion hazard. | [Chemical Properties]
white to grey fine powder | [Physical properties]
Colorless needles; tetragonal structure; density 1.178 g/cm3 at 17.5°C; melts around 375°C; starts to decompose at 320°C; decomposes at 400°C; soluble in cold water; decomposes in hot water; slightly soluble in ethanol and liquid ammonia; insoluble in benzene and ether. | [Uses]
In Claisen condensations, alkylation of nitriles and ketones, synthesis of ethynyl Compounds, acetylenic carbinols. | [Uses]
Lithium amide is used in the preparation of active pharmaceutical ingredients and antioxidants. It acts as a catalyst for polymers, as nucleophiles and as strong bases. It serves as a reagent in the synthesis of antiinflamatory and preoresolving protectin D1, chemotype dipeptidyl peptidase IV inhibitors and sterically congested triarylamines. It finds application in dyes displaying large stokes shifts. In addition to this, it is used as a reagent for cross-coupling of aryl chlorides and amine. | [Uses]
Reagent for synthesis of: Antiinflamatory and preoresolving protectin D11 Chemotype dipeptidyl peptidase IV inhibitors2 Sterically congested triarylamines3 Dyes displaying large Stokes shifts4 GM1 ganglioside derivatives5Reagent for cross-coupling of aryl chlorides and amines6 | [Flammability and Explosibility]
Substances and mixtureswhichincontactwithwateremitflammablegases | [Purification Methods]
Purify it by heating at 400o while NH3 is passed over it in the upper of two crucibles (the upper crucible is perforated). The LiNH2 will drip into the lower crucible through the holes in the upper crucible. The product is cooled in a stream of NH3. Protect it from air and moisture, store it under N2 in a clear glass bottle sealed with paraffin. Store it in small quantities so that all the material is used once the bottle is opened. If the colour of the amide is yellow, it should be destroyed as it is likely to have oxidised and to EXPLODE. On heating above 450o it is decomposed to Li2NH, which is stable up to 750-800o. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 463 1963, Greenlee & Henne Inorg Synth II 135 1953.] |
| Questions And Answer | Back Directory | [Preparation]
Lithium amide is obtained by dissolution of lithium metal in liquid ammonia. The reaction is slow, but may be catalyzed by cobalt nitrate:
2Li + 2NH3 → 2LiNH2 + H2
It also is obtained by passing gaseous ammonia over lithium hydride:
LiH + NH3 → LiNH2 + H2
| [Reactions]
Lithium amide decomposes to imide when heated above 400°C: 2LiNH2 → Li2NH + NH3
It is used in several organic syntheses. Some of these synthetic reactions are based on the mechanism that the terminal alkynes react with the stronger base, the anion, forming the weaker conjugate base:

It converts vic dibromide to bromoalkene and then alkyne:

 Ketones can be converted into alkynes:
Ketones can be converted into alkynes: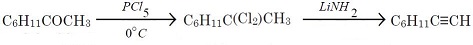
|
|
|