| Identification | More | [Name]
Sulfamethoxypyridazine | [CAS]
80-35-3 | [Synonyms]
MIDICEL
N1-(6-METHOXYPYRIDAZIN-3-YL)SULFANILAMIDE
SULFAMETHOXYPYRIDAZINE
3-(4-Aminobenzenesulfonamido)-6-methoxypyridazine
3-(p-Aminobenzenesulfamido)-6-methoxypyridazine
3-Methoxy-6-sulfanilamidopyridazine
3-Methoxy-6-sulfanylamidopyridazine
3-p-Aminobenzenesulphonamido-6-methoxypyridazine
3-Sulfa-6-methoxypyridazine
3-Sulfanilamide-6-methoxypyridazine
3-Sulfanilamido-6-methoxypyridazine
4-Amino-N-(6-methoxy-3-pyridazinyl)-benzenesulfonamide
6-Methoxy-3-pyridazinylsulfanilamide
6-Methoxy-3-sulfanilamidopyridazine
6-Sulfanilamido-3-methoxypyridazine
Altezol
Benzenesulfonamide, 4-amino-N-(6-methoxy-3-pyridazinyl)-
CL 13494
cl13494
Davosin | [EINECS(EC#)]
201-272-5 | [Molecular Formula]
C11H12N4O3S | [MDL Number]
MFCD00057372 | [Molecular Weight]
280.3 | [MOL File]
80-35-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
182-183° | [Boiling point ]
564.9±60.0 °C(Predicted) | [density ]
1.3936 (rough estimate) | [refractive index ]
1.6200 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Crystalline Powder | [pka]
6.7(at 25℃) | [color ]
White to yellow | [Water Solubility ]
579.5mg/L(25 ºC) | [Merck ]
13,9004 | [BRN ]
277076 | [InChI]
InChI=1S/C11H12N4O3S/c1-18-11-7-6-10(13-14-11)15-19(16,17)9-4-2-8(12)3-5-9/h2-7H,12H2,1H3,(H,13,15) | [InChIKey]
VLYWMPOKSSWJAL-UHFFFAOYSA-N | [SMILES]
C1(S(NC2=NN=C(OC)C=C2)(=O)=O)=CC=C(N)C=C1 | [CAS DataBase Reference]
80-35-3(CAS DataBase Reference) | [NIST Chemistry Reference]
Pyridazine, sulfamethoxy-(80-35-3) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R37/38:Irritating to respiratory system and skin .
R41:Risk of serious damage to eyes. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S39:Wear eye/face protection . | [WGK Germany ]
2
| [RTECS ]
WP0400000
| [F ]
10 | [HS Code ]
29350090 | [Toxicity]
LD50 orally in mice: 1750 mg/kg, (Seki) |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
Antibacterial. | [Application]
Sulfamethoxypyridazine may be used as a reference standard for the determination of sulfamethoxypyridazine in pharmaceutical formulations by liquid chromatography method. | [Definition]
ChEBI: A sulfonamide consisting of pyridazine having a methoxy substituent at the 6-position and a 4-aminobenzenesulfonamido group at the 3-position. | [Indications]
This drug possesses antibacterial activity with respect to a few cocci and colon bacillus. It
is a long-lasting drug. It is used for treating pneumonia, bronchitis, tonsillitis, purulent otitis
and meningitis, purulent infections of the urinary tract, dysentery, and others. Synonyms of
this drug are sulfapyridazine, sufalex, retasulfin, and many others. | [Brand name]
Amidin;Aseptilex;Asey-sulfa;Bimalong;Biocorn;Bio-cron;Bio-pectodil;Davosin suspension;Deltavagin;Desulfon;Durasul jarabe;Elix;Ensulfa;Eusulfa;Exazole;Farinffnicol;Fercasulf;Hesse-sulfon;Ketiak;Kynex acetyl;Lentosulfa;Linder;Logisul jarabe;Longamid;Longisul;Metamit;Metazina;Metuzina;Minikel;Novosulfin;Pirasulfon;Ralenta;Rotardon;S.d.m.;Septotryl;Smop;Sulamin;Sulfa spirig;Sulfabon;Sulfadazina;Sulfadepot;Sulfadin;Sulfadurazin;Sulfaintensa;Sulfakeyn;Sulfametopyridazin;Sulfamizina;Sulfamyd;Sulfapyrazin;Sulfatar;Sulfocidan;Sulfonamid;Sulforetent;Sulfo-rit;Unisulfa dulcis;Uroplex;Velaten;Volocid;Vtg 44. | [World Health Organization (WHO)]
Sulfamethoxypyridazine, a sulfonamide anti-infective agent, was
introduced in 1957 for the treatment of bacterial infections. The importance of
sulfonamides has subsequently decreased as a result of increasing bacterial
resistance and their replacement by antibiotics which are generally more active
and less toxic. The sulfonamides are known to cause serious adverse effects such
as renal toxicity, sometimes fatal exfoliative dermatitis and erythema multiforma
and dangerous adverse reactions affecting blood formation such as
agranulocytosis and haemolytic or aplastic anaemia. Commercial manufacture of
the drug has been discontinued by at least one major manufacturer but supplies
can still be obtained on special request, particularly for patients with dermatitis
herpetiformis in which condition it has been claimed to be beneficial. | [General Description]
Sulfamethoxypyridazine is a sulfonamide antibiotic mainly used to prevent infections as well as conditions such as coccidiosis, diarrhea and gastroenteritis. | [Pharmaceutical Applications]
3-Sulfanilamido-6-methoxypyridazine. Properties are similar
to those of sulfadimethoxine. A rapidly absorbed, long-acting
compound (half-life 38 h) with a high degree of protein binding
(96%). A 1 g oral dose achieves a peak plasma concentration
of around 100 mg/L after 5 h. Its use has been largely
discontinued because of frequent adverse effects, but there are
reports of benefit in dermatitis herpetiformis. It has been used
in combination with trimethoprim. | [Synthesis]
Sulfamethoxypyridazine, N1
-(6-methoxy-3-pyridazinyl)sulfanil�amide (33.1.43), is synthesized by replacing the chlorine atom in 6-chloro-3-(4-aminoben�zenesulfonilamido)pyridazine with a methoxy group in 33.1.42 using sodium methoxide. The
initial 6-chloro-3-(4-aminobenzenesulfonylamido)-pyridazine (33.1.42) is in turn synthesized
by reacting 4-aminobenzenesulfanilamide with easily accessible 3,6-dichloropyridazine.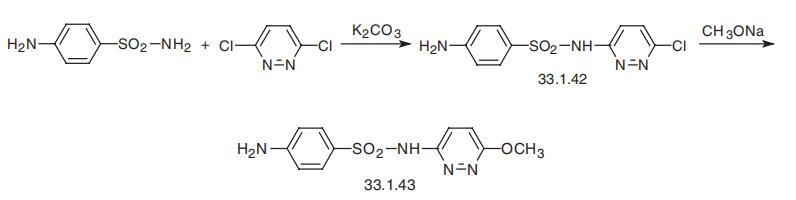
| [storage]
Store at -20°C |
|
|