| Identification | Back Directory | [Name]
Tetryzoline | [CAS]
84-22-0 | [Synonyms]
Visine
Tyzanol
Ocuzolin
TETRYZOLNE
Tetryzolin
TETRYZOLINE
tetrahydrazoline
TETRAHYDROZOLINE
dl-Tetrahydrozoline
Tetryzoline USP/EP/BP
Tetryzoline (tetrahydrozoline)
2-(1,2,3,4-Tetrahydro-1-napthyl)-2-imidazoline
2-(1,2,3,4-Tetrahydro-1-naphthyl)-2-imidazoline
2-Imidazoline, 2-(1,2,3,4-tetrahydro-1-naphthyl)-
2-(1,2,3,4-Tetrahydronaphthalen-1-yl)-2-imidazoline
4,5-Dihydro-2-(1,2,3,4-tetrahydro-1-naphthyl)-1H-imidazole
4,5-Dihydro-2-(1,2,3,4-tetrahydronaphthalen-1-yl)-1H-imidazole
2-(1,2,3,4-Tetrahydro-1-naphthalenyl)-4,5-dihydro-1H-imidazole
1H-Imidazole, 4,5-dihydro-2-(1,2,3,4-tetrahydro-1-naphthalenyl)-
2-Imidazoline, 2-(1,2,3,4-tetrahydro-1-naphthyl)- (6CI, 7CI, 8CI)
1H-Imidazole, 4,5-dihydro-2-(1,2,3,4-tetrahydro-1-naphthalenyl)- (9CI) | [EINECS(EC#)]
201-522-3 | [Molecular Formula]
C13H16N2 | [MDL Number]
MFCD00216026 | [MOL File]
84-22-0.mol | [Molecular Weight]
200.28 |
| Hazard Information | Back Directory | [Originator]
Tyzine,Pfizer,US,1954 | [Uses]
Tetrahydrozoline is generally used in the form of eye drops for constriction of blood ves�sels as well as locally for minor inflammations and bites. | [Definition]
ChEBI: Tetryzoline is a member of imidazolines and a carboxamidine. It has a role as a sympathomimetic agent and a nasal decongestant. It is a conjugate base of a tetryzoline(1+). | [Manufacturing Process]
A mixture of 540 grams (9.0 mols) of ethylenediamine, 270 grams (1.53
mols) of 1,2,3,4tetrahydro-α-naphthoic acid, and 360 ml (4.32 mols) of
concentrated hydrochloric acid was introduced into a two-liter, three-necked
flask fitted with a thermometer, stirrer, and distillation takeoff. The mixture
was distilled under a pressure of about 20 mm of mercury absolute until the
temperature rose to 210°C. Thereafter, heating was continued under
atmospheric pressure and when the temperature reached about 260°C, an
exothermic reaction was initiated. The heat was then adjusted to maintain a
reaction temperature of 275° to 280°C for 45 minutes and the mixture
thereafter cooled to room temperature.
900 ml of 4 N hydrochloric acid was added and the aqueous layer stirred with
warming until a clear, brown solution resulted. This brown solution was made
strongly alkaline with sodium hydroxide. The oil that separated solidified and
was collected on a filter leaving filtrate A. The solid was dissolved in 370 ml of
alcohol with warming, and the solution was treated with 130 ml of
concentrated hydrochloric acid with stirring and cooling. This acidified mixture
was diluted with 300 ml of ether and chilled. The solid salt was collected and
dried and the filtrate concentrated to approximately 300 ml, diluted with 300
ml of ether and the salt which separated collected and dried.
Filtrate A was extracted with ether, dried, acidified with alcoholic hydrogen
chloride, and the salt which separated was collected and dried. There was
thus obtained, when all the salt had been combined, 250 grams (69.3% of the
theoretical yield) of 2-(1,2,3,4-tetrahydro-1-naphthyl)imidazoline
hydrochloride, melting at 256° to 257°C. | [Therapeutic Function]
Nasal decongestant, Pharmaceutic aid | [Synthesis]
Tetrahydrozoline, 2-(1,2,3,4-tetrahydro-1-naphthalenyl)-2-imidazoline
(11.1.41), is synthesized in one step by the heterocyclization of 1-cyanotetraline with eth�ylenediamine [45].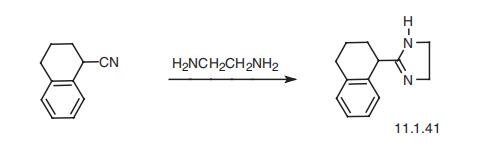
|
|
|