| Identification | More | [Name]
Amodiaquine | [CAS]
86-42-0 | [Synonyms]
4-[(7-chloro-4-quinolinyl)amino]-2-[(diethylamino)methyl]-phenol
7-chloro-4-(3-diethylaminomethyl-4-hydroxyanilino)quinoline
AMODIAQUIN
AMODIAQUINE
4-((7-chloro-4-quinolyl)amino)-alpha-(diethylamino)-o-creso
4-((7-Chloro-4-quinolyl)amino)-alpha-(diethylamino)-o-cresol
4-[(7-Chloro-4-quinolinyl)amino]-alpha-(diethylamino)-o-cresol
7-Chloro-4-(3-diethylaminomethyl-4-hydroxyphenylamino)quinoline
Amodiaquine, ring-closed
CAM-AQ1
CAM-AQI
Camochin
Camoquin
Camoquinal
Camoquine
Flavoquine
Miaquin
o-Cresol, 4-((7-chloro-4-quinolyl)amino)-alpha-(diethylamino)-
Phenol, 4-[(7-chloro-4-quinolinyl)amino]-2-[(diethylamino)methyl]-
Quinoline, 7-chloro-4-((3-((diethylamino)methyl)-4-hydroxyphenyl)amino)- | [EINECS(EC#)]
201-669-3 | [Molecular Formula]
C20H22ClN3O | [MDL Number]
MFCD00552927 | [Molecular Weight]
355.86 | [MOL File]
86-42-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Cyrstalline Solid | [Melting point ]
208°C | [Boiling point ]
478.0±45.0 °C(Predicted) | [density ]
1.258 | [storage temp. ]
-20°C Freezer | [solubility ]
DMSO (Slightly, Sonicated), Methanol (Slightly) | [form ]
Solid | [pka]
9.43±0.50(Predicted) | [color ]
Pale Yellow to Light Yellow | [Usage]
An antimalarial | [CAS DataBase Reference]
86-42-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Amodiaquine(86-42-0) |
| Hazard Information | Back Directory | [Description]
Amodiaquine is a prodrug form of the antimalarial compound N-desethyl amodiaquine (Item No. 20822).1,2 It is active against several strains of P. falciparum in vitro (EC50s = 0.23-0.52 nM) and exhibits a synergistic effect when used in combination with N-desethyl amodiaquine.1 Amodiaquine dose-dependently inhibits development of parasitemia in a mouse model of P. berghei infection.3 | [Chemical Properties]
Cyrstalline Solid | [Originator]
Camoquin HCl,Parke Davis,US,1950 | [Uses]
An antimalarial | [Definition]
ChEBI: A quinoline having a chloro group at the 7-position and an aryl amino group at the 4-position. | [Indications]
Amodiaquine (Camoquin) is another 4-aminoquinoline
derivative whose antimalarial spectrum and adverse reactions
are similar to those of chloroquine, although
chloroquine-resistant parasites may not be amodiaquine-
resistant to the same degree. Prolonged treatment
with amodiaquine may result in pigmentation of
the palate, nail beds, and skin. There is a 1:2000 risk of
agranulocytosis and hepatocellular dysfunction when
the drug is used prophylactically. | [Manufacturing Process]
72.8 g (0.5 mol) of p-aminophenol hydrochloride is dissolved in 500 cc of
water and added to 99 g (0.5 mol) of 4,7-dichloroquinoline. After a few
minutes of warming in a steam bath, 4-(4'-hydroxyanilino)-7-chloroquinoline
hydrochloride, of sufficient purity for use in further experiments, precipitates
as a yellow crystalline solid. Recrystallized from methanol, the MP is over
300°C.
A mixture consisting of 13.5 g of 4-(4'-hydroxyanilino)-7-chloroquinoline
hydrochloride dissolved in absolute ethanol is treated with a solution of 4.38 g of diethylamine and 1.8 g of paraformaldehyde in 20 cc of absolute ethanol.
The reaction mixture is heated under reflux for 16 hours, evaporated to onehalf
volume and the warm solution treated with an excess of hydrogen
chloride dissolved in absolute ethanol. Acetone is added to the warm solution
until it becomes turbid and then the solution is cooled. The crude
dihydrochloride which separates is collected and purified by recrystallization
from methanol; MP 240-242°C.
By using an equivalent amount of 4-(4'-hydroxyanilino)-7-bromoquinoline in
the above procedure, 4-(3'-diethylaminomethyl-4'-hydroxyanilino)-7-
bromoquinoline dihydrochloride is obtained; MP (base) 206-208°C dec. | [Brand name]
Camoquin (Parke-Davis);Amodoquin tablets;Basoquin;Caniquin. | [Therapeutic Function]
Antimalarial | [World Health Organization (WHO)]
Amodiaquine, an antimalarial agent related to chloroquine, was
introduced over 40 years ago for the treatment and prophylaxis of malaria. The
drug was voluntarily withdrawn in the United Kingdom in 1975 for commercial
reasons but was subsequently reintroduced in 1985 to meet the medical demand
for an antimalarial drug to deal with the rapid spread of chloroquine-resistant
falciparum malaria in Asia and Africa. By 1986 a significant number of cases of
agranulocytosis associated with prophylactic use, some of which were fatal, had
been reported there and it has been estimated that the frequency of this risk is of
the order of 1:2,000. Although most cases occurred when amodiaquine had been
used in combination with other antimalarials, the major manufacturer decided to
withdraw the prophylactic indication worldwide following discussions with experts.
Preparations remain available for the treatment of acute attacks of malaria which
involves only a short period of exposure to the drug.
(Reference: (WHODI) WHO Drug Information, 1, 5, 1987) | [Pharmaceutical Applications]
A mono-Mannich-base 4-aminoquinoline, formulated
as the dihydrochloride dihydrate or free base for oral
administration.
It is active against P. falciparum and P. vivax and is more
active than chloroquine for the treatment of uncomplicated P.
falciparum malaria. Chloroquine-resistant strains may remain
susceptible, but resistance to amodiaquine is also spreading
in some regions of Africa. The pharmacological properties are
similar to those of chloroquine. The terminal elimination halflife
is 1–3 weeks. It is rapidly and extensively metabolized to the
desethyl derivative which has reduced antiplasmodial activity.
Prophylactic use has been abandoned because of agranulocytosis
and hepatotoxicity due to formation of a quinoneimine
metabolite.
A fixed dose combination with artesunate and derivatives
(for example, isoquine) with altered metabolism and reduced
toxicity is in development. | [Clinical Use]
Mechanistically, it is very similar to chloroquine and does nothave any advantages over the other 4-aminoquinoline drugs.When used for prophylaxis of malaria, it had a higher incidenceof hepatitis and agranulocytosis than that was chloroquine.There is evidence that the hydroquinone (phenol)amine system readily oxidizes to a quinone imine either autoxidatively and/or metabolically, and this productmay contribute to amodiaquine’s toxicity. | [Clinical Use]
Treatment of falciparum malaria | [Synthesis]
Amodiaquin, 4-[(7-chloro-4-quinilyl)amino]-|á-diethylmaino-o-cresol
(37.1.1.21), is made by reacting 4,7-dichloroquineoline (37.1.1.1) with 4-aminophenol to make 7-chloro-4-(4-hydroxyphenylamino)-quiniline (37.1.1.20), which then undergoes an
aminomethylation reaction using formaldehyde and diethylamine, giving amodiaquin.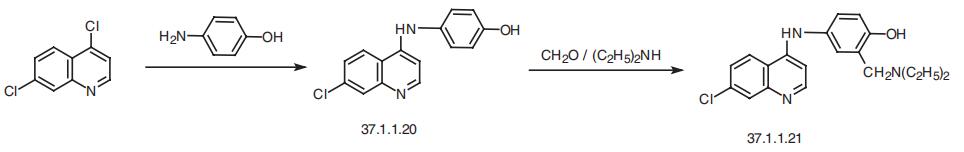
| [Purification Methods]
Amodiaquin crystallises from 2-ethoxyethanol or EtOH. [Burckhalter et al. J Am Chem Soc 70 1363 1948, Beilstein 22 III/IV 4647.] |
|
|