| Identification | Back Directory | [Name]
Eluxadoline | [CAS]
864821-90-9 | [Synonyms]
EOS-61916
Eluxadolin
Eluxadoline
JNJ 27018966
API-Diarrhea
Eluxadoline(JNJ 27018966)
5-(((S)-2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)-N-((S)-1-(5-phenyl-1H-imidazol-2-yl)ethyl)propanamido)methyl)-2-methoxybenzoic acid
5-(((S)-2-amino-3-(4-carbamoyl-2,6-dimethylphenyl)-N-((S)-1-(4-phenyl-1H-imidazol-2-yl)ethyl)propanamido)methyl)-2-methoxybenzoic acid | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C32H35N5O5 | [MDL Number]
MFCD28386164 | [MOL File]
864821-90-9.mol | [Molecular Weight]
569.65 |
| Chemical Properties | Back Directory | [Melting point ]
>180°C (dec.) | [Boiling point ]
834.2±65.0 °C(Predicted) | [density ]
1.284±0.06 g/cm3(Predicted) | [storage temp. ]
Refrigerator | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
4.01±0.10(Predicted) | [color ]
White to Off-White | [InChIKey]
QFNHIDANIVGXPE-FNZWTVRRSA-N | [SMILES]
C(O)(=O)C1=CC(CN(C(=O)[C@@H](N)CC2=C(C)C=C(C(N)=O)C=C2C)[C@H](C2NC(C3=CC=CC=C3)=CN=2)C)=CC=C1OC | [CAS DataBase Reference]
864821-90-9 |
| Hazard Information | Back Directory | [Definition]
ChEBI: An amino acid amide obtained by the formal condensation of the carboxy group of 4-carbamoyl-2,6-dimethyl-L-phenylalanine with the secondary amino group of 2-methoxy-5-({[(1S)-1-(4-phenylimidazol-2-yl)ethyl]amino}methyl)ben
oic acid. It has mixed opioid receptor activity and is used for treatment of irritable bowel syndrome with diarrhoea. | [Description]
Eluxadoline, originally developed
by Janssen and currently marketed by Allergan (formerly
Actavis), was approved in May 2015 by the FDA for the
treatment of diarrhea-predominant irritable bowel syndrome
(IBS-D). Eluxadoline, an orally dosed agent, employs a
unique mechanism for IBS-D treatment, as it functions
simultaneously as a μ- and κ-opioid receptor agonist and a δ-
opioid receptor antagonist, leading to a first-in-class therapy
for treatment of IBS-D. Specifically, in animal studies,
eluxadoline was found to interact with opioid receptors in the
gut, inhibiting neurogenically mediated secretion and reducing
intestinal contractility. Additionally, the treatment led to a
decrease in stress-induced acceleration of upper GI transit
without causing rebound constipation, earning its mark as
a first-line therapeutic treatment for IBS-D. In two phase III
clinical trials of over 2400 patients with IBS-D, patients taking
eluxadoline showed a greater improvement toward the end
point (≥30% improvement from their baseline IBS-D score on
at least 50% of days treated with eluxadoline) compared to
patients treated with placebo. | [Uses]
Eluxadoline is an orally-active drug used to reduce symptoms of irritable bowel syndrome such as diarrhea and abdominal pain. | [Pharmacokinetics]
Following oral administration of 100 mg VIBERZI in healthy subjects, the Cmax of eluxadoline was approximately 2 to 4 ng/mL, and AUC was 12 to 22 ng. h/mL. Eluxadoline has approximately linear pharmacokinetics with no accumulation upon repeated twice-daily dosing. The variability of eluxadoline pharmacokinetic parameters ranges from 51% to 98%.
Absorption: The absolute bioavailability of eluxadoline has not been determined. The median Tmax value was 1.5 hours (1 to 8 hours) under fed conditions and 2 hours (range: 0.5 to 6 hours) under fasting conditions.
The administration of VIBERZI with a high-fat meal that contained approximately 800 to 1000 total calories, with 50% of calories derived from fat content, decreased the Cmax of eluxadoline by 50% and AUC by 60%.
Distribution: Plasma protein binding of eluxadoline was 81%.
Elimination: The mean plasma elimination half-life of eluxadoline ranged from 3.7 hours to 6 hours.
Metabolism: The metabolism of eluxadoline is not established. There is evidence that glucuronidation can occur to form an acyl glucuronide metabolite.
Excretion: Following a single oral dose of 300 mg [14C] eluxadoline in healthy male subjects, 82.2% of the total radioactivity was recovered in feces within 336 hours and less than 1% was recovered in urine within 192 hours.
| [Clinical Use]
Mixed mu-opioid receptor agonist, kappa-opioid receptor
agonist, and a-delta opioid receptor antagonist:
Treatment of irritable bowel syndrome with
diarrhoea | [Synthesis]
The synthesis of eluxadoline begins with preparation of
advanced coupling component 85, which could be completed
via a four-step route from commercially available N-Bocprotected
aminoester 83 . Triflate formation using N-phenyltrifluoromethanesulfinimide in DCM under
basic conditions led to nearly quantitative yield of the desired
triflate, which was subjected to a carbonylation reaction to yield
aryl acid 84 in 94% yield. Employing NH4Cl as a source of
ammonia, amidation of 84 took place in the presence of
PyBOP/HOBt and DIPEA in DMF. Finally, acid 85 was
revealed upon methyl ester saponification with aqueous LiOH
in THF. This sequence provided 85 without purification.
With coupling component 85 in hand and initiated from a HOBt and EDC?¤HCl-mediated coupling of commercial
N-Cbz-L-alanine (86) with commercial 2-amino acetophenone
hydrochloride (87) to provide intermediate 88 in 83%
yield. Addition of NH4OAc and AcOH to a suspension
of 88 in refluxing xylenes furnished the desired imidazole in
excellent yield (95%). Submission of this N-Cbz-imidazole to
hydrogenation conditions (H2, Pd/C, MeOH) enabled
liberation of the free amine to access 89 in quantitative yield
following filtration and concentration. From intermediate 89,
reductive amination with commercially available aryl aldehyde
90 under standard conditions (NaBH4, MeOH) followed by
subsequent coupling of the corresponding crude amine with acid 85 using HOBt/EDC?¤HCl enabled formation of the
carbon framework of eluxadoline (91). Saponification of the
ester within 91 with LiOH in MeOH/THF yielded the
corresponding acid in quantitative yield. Immediate subjection
of this intermediate to acidic conditions (HCl in EtOAc/THF)
led to N-Boc cleavage and isolation of eluxadoline (XII) as the
bis-HCl salt in 71% yield, requiring no further purification.
It should be noted that since this initial report, additional
details for the isolation of eluxadoline in high purity in various
crystal forms and as a zwitterion have been reported,66 although
most reported routes described isolation of this drug in its HCl
salt form.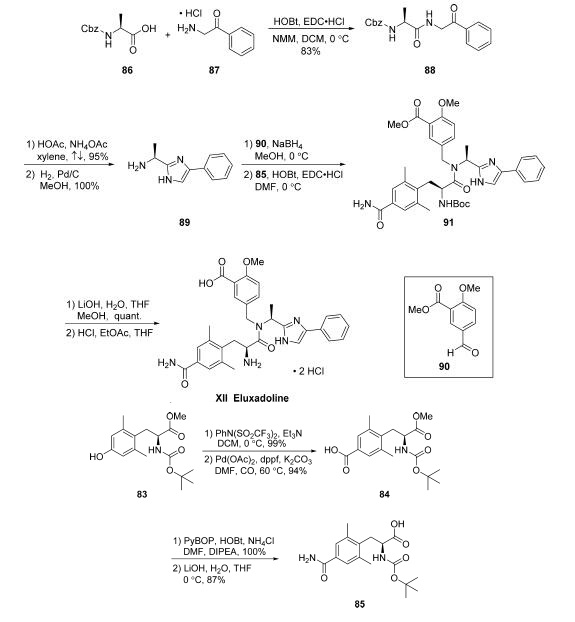
| [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
rifampicin - avoid.
Antivirals: concentration possibly increased by
atazanavir, lopinavir, ritonavir, saquinavir and
tipranavir - avoid.
Ciclosporin: concentration of eluxadoline increased
- avoid.
Lipid-lowering agents: concentration possibly
increased by gemfibrozil - avoid. | [Metabolism]
Eluxadoline is mainly excreted in the faeces, either as
unabsorbed active substance or via the biliary system with
the kidney playing a minimal role in elimination. |
| Questions And Answer | Back Directory | [Overview and History]
Eluxadoline is a mixed mu-opioid receptor agonist, kappa-opioid receptor agonist, and a-delta opioid receptor antagonist that can be used for the treatment of diarrhea-predominant irritable bowel syndrome(IBS-D)[1-4]. The mu-, kappa-, and delta-opioid receptors participating in regulating endogenous and exogenous opioid response in the central nervous system and peripherally in the gastrointestinal system. The agonism of peripheral mu-opioid receptors results in reduced colonic motility, while antagonism of central delta-opioid receptors results in improved analgesia, making eluxadoline usable for the symptoms of both pain and diarrhea characteristic of IBS-D[1, 4].
Eluxadoline is marketed under the tradename Viberzi(FDA) in US(at may 2015)[1, 2, 4, 5]. It is an antimotility agent that decreases bowel contractions, inhibits colonic transit, and reduces ?uid/ion secretion resulting in improved symptoms of abdominal pain and reductions in the Bristol Stool Scale. It is also marketed in Europe under the trade name of “Truberzi”[3].
| [Indication and applications]
Eluxadoline is indicated for the treatment of diarrhea-predominant irritable bowel syndrome(IBS-D). It can work directly in the intestines to slow the movement of food during digestion. Eluxadoline also makes the nerves in the intestines less sensitive to stimulation[1, 6].
| [Mode of action]
Eluxadoline takes effect through acting as the antagonist of mu-opioid receptor agonis, kappa opioid receptor agonist and a delta opioid receptor[7,8]. Eluxadoline can be used for diarrhea predominant IBS because it reduces intestinal contractility and normalizes stress-induced acceleration of upper GI transit[9]. Antagonistic activity at the delta receptor minimizes the constipating effect usually seen by mu-opioid receptor agonists alone. Because of it's limited systemic bioavailability, there may be less side effects associated with the use of eluxadoline in comparison with other therapies used to treat diarrhea predominant IBS[10].
| [Adverse reactions]
Common adverse reaction associated with Eluxadoline include constipation, nausea, abdominal pain, upper respiratory tract infection, pancreatitis, sphincter of Oddi spasm, hypersensitivity reactions, vomiting, nasopharyngitis, abdominal distention, bronchitis, dizziness, flatulence, rash, increased ALT, fatigue, viral gastroenteritis and stomach pain[4, 6, 11]. Less common adverse reactions also include gastroesophageal reflux disease, sedation, somnolence, and euphoric mood, respiration disorders such as asthma, bronchospasm, respiration failure and wheezing. Constipation was the most commonly reported adverse reaction in during the treatment by Eluxadoline. Approximately 50% of constipation events occurred within the first 2 weeks of treatment while the majority occurred within the first 3 months of therapy. Similar rates of constipation occurred between the active and placebo arms beyond 3 months of treatment. In severe cases, constipation can even lead to permanent discontinuation of drugs[11].
| [Warning and Precaution]
Eluxadoline is not approved for use by anyone younger than 18 years old[6].
Eluxadoline is contradicted for administration in patients who suffer from a pancreas disorder, severe liver disease, alcoholism, and a blockage in the intestines, a gallbladder disorder, or those whose gallbladder has been removed[12].
People who are allergic to eluxadoline should also be disabled for using. In addition, it is also not allowed for patients who have a history of gallbladder, obstruction digestive problems caused by a muscle valve called the sphincter of Oddi(SFINK-ter of OD-dee), pancreas disorder, severe liver disease, a habit of drinking more than 3 alcoholic beverages per day[12, 13].
To make sure eluxadoline is safe for you; tell your doctor if you have liver disease.
It is not known whether this medicine will harm an unborn baby. Tell your doctor if you are pregnant or plan to become pregnant. It is not known whether eluxadoline passes into breast milk or if it could harm a nursing baby. Tell your doctor if you are breast-feeding a baby[12].
| [References]
- https://www.drugbank.ca/drugs/DB09272
- "Viberzi[eluxadoline] Tablets, for Oral Use, CIV. Full Prescribing Information". Actavis Pharma, Inc. Parsippany, NJ 07054 USA. Retrieved 26 December 2015.
- "Truberzi". European Medicines Agency. 29 September 2016.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206940s000lbl.pdf
- Cash, B. D. "Eluxadoline: a promising therapy that raises many questions. " Translational Gastroenterology & Hepatology 1(2016]: 76.
- https://www.everydayhealth.com/drugs/eluxadoline
- Fujita, Wakako, et al. "Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers." Biochemical Pharmacology 92.3(2014]: 448-456.
- Levycooperman, N, et al. "Abuse Potential and Pharmacodynamic Characteristics of Oral and Intranasal Eluxadoline, a mixed μand κ-Opioid Receptor Agonist and δ-Opioid Receptor Antagonist. " Journal of Pharmacology & Experimental Therapeutics 359.3(2016]: 471-481.
- Chen, Hong Yu, J. J. Feng, and T. T. Song. "Eluxadoline, a new drug for treating diarrhea-predominant irritable bowel syndrome." Chinese Journal of New Drugs[2017].
- Davenport JM, Covington P, Bonifacio L, McIntyre G, Venitz J: Effect of uptake transporters OAT3 and OATP1B1 and efflux transporter MRP2 on the pharmacokinetics of eluxadoline. J Clin Pharmacol. 2015 May; 55(5]: 534-42. doi: 10.1002/jcph.442. Epub 2015 Jan 14.
- https://www.rxlist.com/viberzi-drug.htm
- https://www.drugs.com/viberzi.html
- Gawron, A. J., and K. Bielefeldt. "Risk of Pancreatitis Following Treatment of Irritable Bowel Syndrome with Eluxadoline." Clinical Gastroenterology & Hepatology the Official Clinical Practice Journal of the American Gastroenterological Association 16.3(2017].
|
|
|