| Identification | More | [Name]
Axitinib | [CAS]
319460-85-0 | [Synonyms]
AXITINIB
Benzamide, N-methyl-2-[[3-[(1E)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]-
N-METHYL-2-{[3-((E)-2-PYRIDIN-2-YLVINYL)-1H-INDAZOL-6-YL]SULFANYL}BENZAMIDE
N-Methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide
AVERMECTINB
Axitinib for research
AG 013736
N-Methyl-2-[[3-[(1E)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]benzamide | [EINECS(EC#)]
638-771-6 | [Molecular Formula]
C22H18N4OS | [MDL Number]
MFCD09837898 | [Molecular Weight]
386.47 | [MOL File]
319460-85-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Solid | [Melting point ]
213-215°C | [Boiling point ]
668.9±55.0 °C(Predicted) | [density ]
1.4 | [storage temp. ]
room temp | [solubility ]
DMSO: ≥8mg/mL | [form ]
powder | [pka]
12.70±0.40(Predicted) | [color ]
white to tan | [Usage]
A tyrosine kinase inhibitor; used in cancer therapy. | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. | [InChI]
InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | [InChIKey]
RITAVMQDGBJQJZ-FMIVXFBMSA-N | [SMILES]
C(NC)(=O)C1=CC=CC=C1SC1=CC2=C(C=C1)C(/C=C/C1=NC=CC=C1)=NN2 | [CAS DataBase Reference]
319460-85-0(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
In January 2012, the US FDA approved axitinib (319460-85-0) (also referred to as
AG-013736) for the treatment of advanced renal cell carcinoma (RCC)
for patients who have not responded to prior therapy.
Axitinib is a pan VEGF inhibitor and functions by binding to
the intracellular tyrosine kinase catalytic domain of VEGF leading to blockade of signaling through this angiogenic pathway. Axitinib is�50–400 times more potent for VEGF (enzyme Ki and cellular IC50s for VEGF 1, 2, and 3 are ~0.1 nM) than first-generation inhibitors like sorafenib and sunitinib. Axitinib also inhibits c-Kit and PDGFR(α/β) with enzyme Ki's of
~2 nM and was selective when tested against a broad panel of other protein
kinases. Axitinib was discovered by a structure-based drug design approach
and binds to the kinase domain of VEGF in a DFG-out conformation.
Axitinib blocks VEGF-2 phosphorylation up to 7 h postdose in vivo and
inhibits endothelial cell proliferation in xenograft tumors implanted in
mice. Synthetic routes to axitinib employing a Migita coupling to form
the diaryl sulfide and a Heck reaction to install the 2-styrylpyridine moiety have been reported.
| [Chemical Properties]
Off-White Solid | [Originator]
Pfizer (United States) | [Characteristics]
Class: receptor tyrosine kinase
Treatment: RCC
Oral bioavailability = 58%
Elimination half-life = 2.5–6.1 h
Protein binding = 99%
| [Uses]
A tyrosine kinase inhibitor; used in cancer therapy. | [Uses]
Axitinib (AG-013736) is a multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFRβ and c-Kit with IC50 of 0.1 nM, 0.2 nM, 0.1-0.3 nM, 1.6 nM and 1.7 nM, respectively. | [Uses]
Axitinib is a multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFRβ and c-Kit with IC50 of 0.1 nM, 0.2 nM, 0.1-0.3 nM, 1.6 nM and 1.7 nM, respectively. | [Uses]
Axitinib is a tyrosine kinase inhibitor. Axitinib is used in cancer therapy. | [Definition]
ChEBI: An indazole substituted at position 3 by a 2-(pyridin-2-yl)vinyl group and at position 6 by a 2-(N-methylaminocarboxy)phenylsulfanyl group. Used for the treatment of advanced renal cell carcinoma after failure of a first line systemic tr
atment. | [Brand name]
Inlyta | [Biochem/physiol Actions]
Axitinib (AG-013736) is an orally available, potent (picomolar) and selective tyrosine kinase inhibitor that blocks VEGF receptors 1, 2 and 3. The drug blocks VEGF-mediated endothelial cell survival, tube formation, and downstream signaling through endothelial nitric oxide synthase, Akt and extracellular signal-regulated kinase. | [Clinical Use]
Sold under the brand name Inlyta® by Pfizer, Inc., axitinib was approved by the FDA in January 2012
for the treatment of advanced renal cell carcinoma (RCC), specifically after the failure of other systemic treatments. Axitinib slows cancer cell proliferation by inhibition of the vascular endothelial growth
factor (VEGF)/VEGF receptor tyrosine (RTK) signaling pathway. In particular, axitinib is a potent
inhibitor of VEGF/RTK 1-3, which selectively slows angiogenesis, vascular permeability, and blood
flow in solid tumors. | [Side effects]
The side effects that you should report to your doctor include:
allergic reactions like skin rash, itching or hives, swelling of the face, lips, or tongue;
high blood pressure;
seizures;
signs and symptoms of bleeding such as bloody or black, tarry stools; red or dark-brown urine; spitting up blood or brown material that looks like coffee grounds; red spots on the skin; unusual bruising or bleeding from the eye, gums, or nose;
signs and symptoms of a blood clot such as breathing problems; changes in vision; chest pain; severe, sudden headache; pain, swelling, warmth in the leg; trouble speaking; sudden numbness or weakness of the face, arm, or leg;
stomach pain.
The side effects that usually do not require medical attention include constipation cough, diarrhea, loss of appetite, nausea, and vomiting.
| [Synthesis]
Numerous patents and papers have been disclosed on the synthesis of
axitinib, a recently published manuscript details the development of the manufacturing route, and
this route is depicted in the scheme. The synthesis began with Migita coupling of commercial iodide 17
with thiophenol 18. Interestingly, this transformation?ˉs efficiency relied upon attention to the number of
equivalents of base and an inert atmosphere in the reaction vessel, conditions which minimized catalyst
poisoning during the reaction. Without isolation, indazole 19 was iodinated to afford diarylthioether 20
in 85-90% yield over the two steps. Protection of the indazole within 20 as its acetamide preceeded a
Heck reaction with 2-vinylpyridine, and then subsequent removal of the indazole protection followed by
a series of recrystallizations yielded axitinib (IV) in a combined 62% yield over the final 4 steps.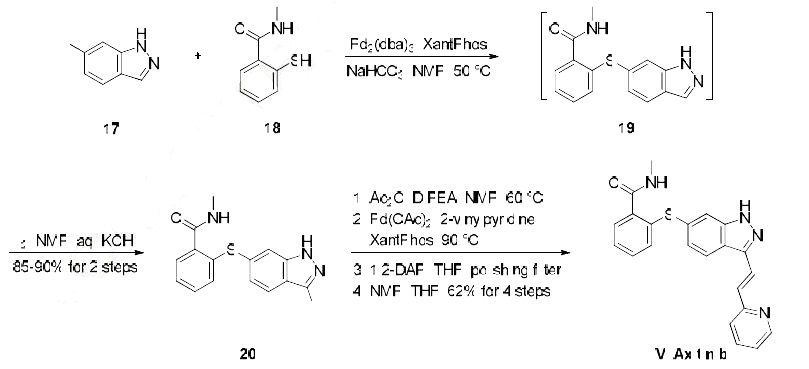
| [Drug interactions]
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis); avoid with pimozide.
Concomitant use with strong CYP3A4/5 inhibitors:
avoid; however, if concomitant use cannot be avoided
then reduce the dose of axitinib by approximately
half; subsequent doses can be increased or decreased
based on individual safety and tolerability; if
CYP3A4/5 inhibitor is discontinued, then increase
the axitinib dose used prior to initiation of the
strong inhibitor after 3-5 half-lives of the inhibitor
(strong CYP3A4/5 inhibitors include ketoconazole,
itraconazole, clarithromycin, atazanavir, indinavir,
ritonavir, saquinavir, and voriconazole). | [Metabolism]
Axitinib is metabolised primarily in the liver by
CYP3A4/5 and to a lesser extent by CYP1A2,
CYP2C19, and UGT1A1.
Most of the drug is excreted via the faeces and urine as
metabolites. | [storage]
Store at +4°C | [References]
1) Hu-Lowe?et al.?(2008),?Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1,2,3; Cancer Res.,?14?7272
2) Ma and Waxman (2008),?Modulation of the antitumor activity of metronomic cyclophosphamide by the angiogenesis inhibitor axitinib; Mol. Cancer Ther.,?7?79
3) Pemovska?et al.?(2015),?Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation; Nature,?519?102
4) Rixe?et al.?(2007),?Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer; a phase II study; Lancet Oncol.,?8?975
5) Yuan?et al.?(2014),?Axitinib augments antitumor activity in renal cell carcinoma via STAT3-dependent reversal of myeloid-derived suppressor cell accumulation;?Biomed.Pharmacother.?68?751
6) Zhang?et al.?(2014),?Axitinib, a selective inhibitor of vascular endothelial growth factor receptor, exerts an anticancer effect in melanoma through promoting antitumor immunity;?Anticancer Drugs?25?204 |
| Questions And Answer | Back Directory | [FDA approved axitinib use of treating advanced kidney cancer]
January 27, 2012, the FDA approved axitinib for the treatment of advanced kidney cancer (renal cell carcinoma) which other drugs unanswer . Inlyta is manufactured and sold by Pfizer,and is a oral pill taken twice a day.
Renal cell carcinoma is a type of tumor originating from the tubular endothelial cells. Axitinib can prevent certain protein called kinases playing a role in tumor growth and metastasis .
Axitinib is a small molecule tyrosine kinase inhibitor, effective against multiple targets, including VEGF receptors 1, 2 and 3.
Dr. Richard Pazdur, hematology and oncology drugs office director of FDA Drug Evaluation and Research Centre, said: "This is the seven kind of drugs allowed treating metastatic or advanced renal cell carcinoma since 2005 . Overall, during this time ,record level of drug development has dramatically changed the treatment of metastatic renal cell carcinoma paradigm, and offers a variety of treatment options for patients. "
In recent years, the drug has been approved for the treatment of kidney cancer include sorafenib (2005), sunitinib (2006), temsirolimus(2007), everolimus (2009), bevacizumab(2009) and pazopanib(2009).
The above information is edited by the chemicalbook of Tian Ye.
| [Binding Mode]
In the co-crystal structure of axitinib bound to VEGFR2 DFG-out inactive conformation, the indazole scaffold forms two hydrogen bonds with the hinge: one between the indazole NH and the backbone carbonyl of Glu917 and the other between the indazole nitrogen the amide NH backbone of Cys919. The styryl group penetrates through the narrow tunnel and extends toward the solvent front. The phenyl sulfide is positioned slightly higher and deeper into the back pocket as compared to other type II inhibitors. The carboxamide forms one H-bond to the NH backbone of Asp1046 and a second H-bond to the carboxylate side chain of Glu885. The indazole head group substantially complements the full length of the channel, contributing to the high affinity with both polar charge stabilization and hydrophobic interactions. It has also been shown that axitinib binds (in a different conformation from the VEGFR2 binding) to the BCR-ABL fusion protein, specifically inhibiting the drug-resistant T315I mutant isoform.
|
|
|