| Identification | More | [Name]
4-Nitrophthalimide | [CAS]
89-40-7 | [Synonyms]
4-NITRO-1,2-BENZENE DICARBOXYLIC ACID, IMIDE
4-NITROPHTHALIMIDE
5-NITRO-1,3-DIHYDRO-2H-ISOINDOL-1,3-DIONE
5-NITRO-1H-ISOINDOLE-1,3(2H)-DIONE
5-NITROISOINDOLE-1,3-DIONE
5-NITROISOINDOLINE-1,3-DIONE
5-NITROPHTHALIMIDE
AKOS B028733
PHTHALIMIDE, 4-NITRO-
P-NITRO-PHTHALIMIDE
1H-Isoindole-1,3(2H)-dione, 5-nitro-
4-nitro-phthalimid
5-nitro-1h-isoindole-3(2h)-dione
4-Nitrophtalimide
4-Nirtophthalimide
5-Nitro-2H-isoindole-1,3-dione | [EINECS(EC#)]
201-905-5 | [Molecular Formula]
C8H4N2O4 | [MDL Number]
MFCD00005884 | [Molecular Weight]
192.13 | [MOL File]
89-40-7.mol |
| Chemical Properties | Back Directory | [Appearance]
slight yellow powder | [Melting point ]
206 °C
| [Boiling point ]
328.09°C (rough estimate) | [density ]
1.5513 (rough estimate) | [refractive index ]
1.4900 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [pka]
7.79±0.20(Predicted) | [color ]
Yellow powder | [Stability:]
Stable. Combustible. Incompatible with moisture, water, strong oxidising agents, strong bases. | [Water Solubility ]
<0.01 g/100 mL at 18 ºC | [BRN ]
180224 | [InChIKey]
ANYWGXDASKQYAD-UHFFFAOYSA-N | [CAS DataBase Reference]
89-40-7(CAS DataBase Reference) | [NIST Chemistry Reference]
4-Nitro-phthalimide(89-40-7) | [EPA Substance Registry System]
89-40-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [RTECS ]
TI5625000 | [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29251900 | [Toxicity]
mmo-sat 333 mg/plate EMMUEG 11(Suppl 12),1,88 |
| Hazard Information | Back Directory | [General Description]
Yellow powder. | [Reactivity Profile]
A nitrated amine. Amines are combustible. The combustion of amines yields noxious NOx. REACTIVITY-Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. | [Air & Water Reactions]
This compound can be hydrolyzed. . Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available, however 4-NITROPHTHALIMIDE is probably combustible. | [Chemical Properties]
slight yellow powder | [Uses]
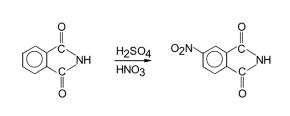
4-Nitrophthalimide is a useful chemical in organic synthesis. Dyes and metabolites. | [Preparation]
4-Nitrophthalimide is synthesized from phthalimide by nitration. Cool the fuming nitric acid to below 5°C, slowly add concentrated sulfuric acid dropwise, and control the temperature to 10-13°C. Plus phthalimide. Leave at room temperature overnight. Then, it was poured into ice water with stirring, and the precipitate was precipitated below 20°C. Filter, wash with water, dry, and then recrystallize from ethanol to obtain 4-nitrophthalimide with a yield of 60%. | [Safety Profile]
Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx. | [Synthesis]
200 mL of sulfuric acid and 50 mL of fuming nitric acid were cooled on an ice
bath and 40 g (0.272 mol) phthalimide was added in portions within 1-1.5 hours so as
not to exceed the internal temperature beyond 10-15 ??C. The mixture was stirred
over ice bath for half an hour and the internal temperature was raised to 35 ??C, and
yellow particles were observed to dissolve. The mixture was stirred for an additional
1 hour, then cooled to 0 ??C again and poured onto 1 kg of ice water mixture. Yellow
4-nitrophthalonitrile precipitated, it was filtered and washed with water until the
filtrate was neutral to blue litmus paper. It was crystallized from 850-900 mL of ethyl
alcohol. Bright yellow crystals were filtered, washed with cold ethyl alcohol, and
dried in a vacuum oven at 80-90 ??C.
 |
|
|