| Identification | Back Directory | [Name]
Collagen | [CAS]
9007-34-5 | [Synonyms]
collagens
Kollagene
COLLAGEN 99%
EHS-collagen
Collagen TypeⅠ
COLLAGEN 99.9%
Collagenpowder
CAPS SIGMAULTRA
Collagensoluble
Collagen Type 4
Collagen ovine
COLLAGEN GRADE A
COLLAGEN GRADE B
COLLAGEN, TYPE 5
COLLAGEN TYPE I, RAT
collagen from rabbit
Collagen hydrolyzate
Collagen polypeptide
COLLAGEN(FROMPIGSKIN)
Collagens polypeptide
COLLAGEN SOLUBLE FORM
FishCollagenPowder95%
HUMAN COLLAGEN TYPE I
HUMAN COLLAGEN TYPE V
HUMAN COLLAGEN TYPE VI
HUMAN COLLAGEN TYPE IV
Collagen from rat tail
HUMAN COLLAGEN TYPE II
NATIVE TYPE 2 COLLAGEN
Collagen I Collagen II
CollagenfromFishscales
NATURE COLLAGEN TYPE II
collagen from calf skin
COLLAGEN BOVINE STERILE
HUMAN COLLAGEN TYPE III
COLLAGEN, TYPE I, HUMAN
BOVINE COLLAGEN TYPE II
COLLAGEN TYPE IV, HUMAN
COLLAGEN TYPE III, HUMAN
COLLAGEN TYPE II, BOVINE
Collagen TypeⅠ, Miscible
Recombinant human Collagen
Collagen dialyzed fraction
ACTIVECOLLAGEN(FROMBIOMASS)
COLLAGEN, TYPE I (RAT TAIL)
collagen from kangaroo tail
collagen from mouse sternum
COLLAGEN, TYPE I, HUMAN SKIN
Collagen from human placenta
Collagen Hydrolysate, Powder
collagen type I from rat tail
COLLAGEN TYPE V (HU PLACENTA)
COLLAGEN TYPE IV (HU PLACENTA)
collagen type I from calf skin
COLLAGEN FROM CALF SKIN TYPE I
Collagen from rabbit skin
Collagen (From Fish) Food Grade
COLLAGEN TYPE III (HU PLACENTA)
COLLAGEN, TYPE I, HUMAN PLACENTA
Collagen, Type V, Human Placenta
COLLAGEN, TYPE IV, HUMAN PLACENTA
collagen from bovine nasal septum
Collagen (From Fish) Cosmetic Grade
COLLAGEN TYPE II, FROM MOUSE STERNUM
collagen type iv from human placenta
collagen from bovine achilles tendon
collagen,type i solution from rat tail
collagen from bovine tracheal cartilage
collagen from chicken sternal cartilage
COLLAGEN ACID SOLUBLE FROM KANGAROOTAIL
COLLAGEN TYPE I FROM CALF SKIN, SOLUBLE
COLLAGEN TYPE I, ACID SOLUBLE FROM HUMAN
COLLAGEN TYPE II (BOV HYALINE CARTILAGE)
Collagen Type IV from human cell culture
Collagen, Type II, Bovine Joint Cartilage
Collagen, Type II, Mouse Sternum Cartilage
COLLAGEN TYPE III, ACID SOLUBLE FROMCALF SKIN
COLLAGEN ACID SOLUBLE FROM BOVINE NASAL SEPTUM
COLLAGEN TYPE II FROM CHICKEN STERNALCAR TILAGE
COLLAGEN TYPE I FROM KANGAROO TAIL ACID SOLUBLE
COLLAGEN TYPE X, ACID SOLUBLE FROMHUMAN PLACENTA
Collagen Solution, 3mg/ml Ultra Pure bovine
COLLAGEN TYPE I FROM RAT TAIL CELLCULTUR E TESTED
COLLAGEN TYPE VI, ACID SOLUBLE FROMHUMAN PLACENTA
COLLAGEN TYPE IV FROM ENGLEBRETH-HOLM-SW ARM MOUSE
COLLAGEN TYPE IX, ACID SOLUBLE FROMHUMAN PLACENTA
Collagen(Insoluble),from Bovine Achilles Tendon
COLLAGEN TYPE I FROM CALF SKIN ACIDSOLUB LE CELL CU
COLLAGEN SOLUTION FROM CALF SKIN(TYPE I) CELL CULT
COLLAGEN TYPE I, ACID SOLUBLE FROM*NEW ZEALAND WHIT
COLLAGEN TYPE IV FROM HUMAN PLACENTAACID SOLUBLE C
COLLAGEN TYPE V, INSOLUBLE FROM BOVINEAC HILLES TEN
COLLAGEN ACID SOLUBLE FROM BOVINETRACHAE L CARTILAG
COLLAGEN TYPE I, INSOLUBLE FROM BOVINEAC HILLES TEN
COLLAGEN TYPE I FROM RAT TAIL, FOR CELL BIOLOGY, SOLUBLE
collagen from engelbreth-holm-swarm murine sarcoma basement membrane
Bovine Collagen Solution Type I, 3 mg/mL, >=95% purity, sterile filtered, BSE-Free, suitable for biomedical research
Bovine Collagen Solution Type I, 6 mg/mL, >=95% purity, sterile filtered, BSE-Free, suitable for biomedical research
COLLAGEN FROM HUMAN PLACENTA BORNSTEIN AND TRAUB TYPE I COLLAGEN FROM HUMAN PLACENTA BORNSTEIN AND TRAUB TYPE I COLLAGE
Bovine Collagen Solution Type I, Acid soluble telocollagen, 6 mg/mL, sterile filtered, BSE-Free, suitable for biomedical research | [EINECS(EC#)]
232-697-4 | [Molecular Formula]
NULL | [MDL Number]
MFCD00163323 |
| Chemical Properties | Back Directory | [Definition]
Protein of connective tissues that comprises approximately 30% of total body protein from which body
tissues are formed. | [storage temp. ]
2-8°C
| [solubility ]
H2O: 5 mg/mL, hazy, colorless and viscous
| [form ]
solution
| [color ]
white to off-white | [PH]
7.0 | [PH Range]
7.0 - 7.6 | [Water Solubility ]
It is soluble in water. | [EPA Substance Registry System]
Collagen(9007-34-5) |
| Questions And Answer | Back Directory | [General description]
Collagen (Collagen) is the main component of the extracellular matrix and mainly presented in the form of insoluble fibrin. It is widely presented in animal bone, tendon, sheath muscles, ligaments, fascia, cartilage and skin, and is a extremely important protein in the connective tissue which accounting for 25% to 30% of the total protein of animal body. Its function are supporting organ and protecting the body. It is also the most important protein specie located in cellular matrix. There are many types of collagen; Collagen of bone and skin generally belong to type I collagen; Collagen of cartilage belongs to type II; Type III collagen is located in embryo skin; Collagen of cell basilar membrane belongs to type IV. Collagen has a unique triple helix structure which makes its molecular structure be very stable and make it have low immunogenicity and good biodegradability property. Collagen, as a natural biological resource, has a unique biocompatible and biodegradable property which cannot be matched by any synthetic polymer material today. In addition, it has other features such as high tensile strength, low immunity, bleeding inhibition and promoting cell growth and proliferation. So, it is widely used in clinical medicine, cosmetics, food, chemicals and many other research areas.
Composition
Collagen is abundant of nutrient. Besides being rich in tryptophan and cysteine, it also ??contains hydroxyproline and pyroglutamatge which is rarely presented in common proteins.
1. In addition to cystine and tryptophan, collagen is rich in 18 kinds of amino acids, including seven kinds of essential amino acids for humans.
2. The content of glycine in collagen accounted for nearly one-third.
3. Collagen contains hydroxylysine and hydroxyproline, which is lacking in other proteins.
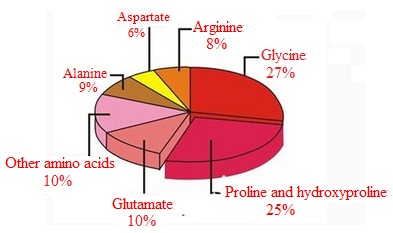
Figure 1 Composition of collagen proteins
| [Structure properties]
In molecular structure, collagen is composed of parallel linear chains, each linear chain consists of three twisted left-handed α-peptide chains which are closely connected by inter-chain interactions and be integrated to form a strong right-handed triple helix (triple-helix) structure. Each α-peptide chain is made up of more than 300 Gly-X-Y tandem repeat with other small segments of different structure connecting the two termini. Amino acid residues in collagen all belong to α-amino acid, wherein X, Y is any amino acid residue other than Gly, but usually for X: Pro; for Y is typically hydroxyproline (Hyp) which is not coded by DNA base. Hydroxyproline is formed by the action of a specific enzyme---proline-4-hydroxylase (prolyl-4-hydroxylase, P4H) on the proline after the formation of primary protein structure. Hydroxy group of hydroxyproline play an important role on stabilizing the helical structure of collagen via the hydrogen bonds. In addition, collagen also contains a certain amount of hydroxylysine (Hyl), which has a similar effect as Hyp. Because the side chains of Pro and Hyp are annular, the bonds between their α-carbon and the amide nitrogen cannot be rotated (The angle is generally fixed at about-60 °). Thereby the high content of these amino acid residues of boosts the formation of α-chain helical structure and further stabilize it. In the Triple-helix structure, GIy group is located in the center of the spiral, and the other amino acids which have a side chain are located outside of the spiral. The helix pitch of right-handed triple helix is 8.55 nm and its radius is 1.5nm. Every lap of each peptide chain contains 30 residues; and the length of every left-handed helical peptide chain is 0.952 nm. Each circle containing about 3.3 residues with the distance between axially adjacent amino acid residues being 0.286nm. Since the triple helix structure is a dislocation structure, the Gly residues come from three peptide chains piled up along the central axis of the helix. In the three-dimensional space, the Gly of chain is adjacent to the X and Y residues of the other two strands, respectively. The Gly in china A is close to the X residue of chain C and the Y residue in chain B. Therefore, the N-H of each Gly residue forms hydrogen bonds with CO of the adjacent X residues. Because hydroxy Hyp residues are also involved in the formation of hydrogen bonds between the chains, the triple helix structure is further stabilized and enhanced. Studies have shown that the presence of divalent iron ions at the N-terminal of collagen polypeptide can further improve the stability of the triple helical structure. In addition, the circular dichroism spectra reveal that polyhydroxy compounds such as sugar alcohols can polymerize with collagens. Molecular mechanics calculations further showed that the polymerization usually occurs in the position where X is Ser of collagen triplets. However, the stability of collagen will be weakened by the increased carbon atoms in such compounds.

Figure 2. The 3D structure of collagen | [Physical and chemical property]
Collagen protein is an amphoteric electrolyte depends on two factors, first, each of the collagen peptide chains having many acidic or basic side groups; Secondly, each of the collagen has α-carboxyl groups and α-amino groups in its two termini. These groups have the ability to accept or give a proton. The dissociable groups can yield either positive or negative charge in particular pH range. In other words, different collagens become ions with many positive charge or negative charge with different medium pH. PKI of the side chain of collagen peptide is slightly different from the PKI value of its amino acid side groups which is due to the influence of neighboring charge in the protein molecule. The isoelectric point of collagen (cowhide) is 7.5 to 7.8, exhibiting a slightly alkaline because the basic amino acid peptide chains of collagen are a little more than an acidic amino acid. As a polymer compound, collagen has colloidal properties and certain viscosity in an aqueous solution with the viscosity being lowest at the isoelectric point. The lower the temperature is, the greater the viscosity becomes.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| [Biological properties]
1. Low immunogenicity
As medical biomaterials, the most important feature of collagen is its low immunogenicity. There are three types of antigen molecules of collagen; the first category is the collagen telopeptide of the non-helical peptide chains; the second is the conformation of the triple helix of collagen, and the third category is the amino acid sequence of a-helix region; wherein the second kind of antigen is only presented in natural collagen molecule; the third kind of antigen only exists in denatured collagen; the first class I antigens factor are presented in both native and denatured collagen.
2. Biocompatibility
Good biocompatibility refers to the well interaction between collagen and host cells and tissues. Whether as the backbone of the new organization before being absorbed, or being assimilated into the host to become part of it, the collagen always has good interaction with the matrix surrounding them which exhibits the coordination of interaction with each other. Moreover, it will become the part of the integral of normal physiological function of cells and tissues.
3. Biodegradability
Collagen can be degraded by specific protease. That means it is biodegradable. Because collagen has a tightening and stable helical structure, the vast majority of protease can only cleave its side chains. Only with specific proteases under certain conditions can the peptide bonds of collagen be broken. Once the collagen peptide bonds were broken, its helical structure is destroyed immediately with the broken collagen polypeptide being totally degraded by protease.
| [Uses]
1. Application in healthcare industry
Collagen is mainly used as scaffolds, skin and bones in tissue engineering. With the application of collagen in tissue engineering, the study of biological membrane also becomes more extensive such as vascular membrane, ligaments and heart valves.
Collagen has good biocompatibility and biodegradable security. It can be degraded and absorbed, and have a good adhesion force thus having a unique skin repair function which can be used for skin transplant after modification. Collagen can form the skeleton of the extracellular matrix and is an important component of cells. It plays a role of anchoring and supporting the cells, and providing the appropriate microenvironment for cell proliferation and growth, also providing a good supply of nutrients. Thus whether as the backbone of the new organization before being absorbed, or being assimilated into the host to become part of it, the collagen always has good interaction with the matrix surrounding them which exhibits the coordination of interaction with each other. This will boost the normal proliferation and repairmen of epithelial cells, thus boosting the healing wound healing of the wound. In this case, it is used for burns and trauma repair, treatment and care, reducing and alleviating the degree of burns. Collagen biological dressing has two advantages: First, the preparation method is simple, easy for sterilization, easy for process; the second is having good adhesion, suitable for the formation of granulation tissue and epithelial cells; can reduce the contracture and antigenic response of wounds, having a good hemostatic effect and easy to absorb tissue exudates.
Because of its unique properties, collagen protein can also be used to treat ophthalmic diseases. Collagen can promote cell growth and the repair of corneal epithelial cell damage, and can also be dissolved in tears. It also has the ability to introduce epithelia cells into the defect area. At the same time, its degraded products can be taken advantage by new cells for new collagen synthesis, which plays the role of connecting different cells. Thus, it is widely applied in the treatment of eye diseases. Collagen can also be used as the carrier for ocular administration, with the function of increasing drug concentration, prolonging drug action time, and reducing the systemic toxicity of the drug, which has broad prospects for development.
In clinical medicine, collagen has also been widely used for cosmetic and orthopedic treatment, for treatment of periodontal diseases, for hard tissue repair, for nerve repair and regeneration, as the wrapping materials of periodontal nerve repair, as artificial organs, as pacemakers and the filling materials of human organs.
2. The application in cosmetics
Collagen can be extracted from animal skin which also contains some proteoglycans like hyaluronic acid, chondroitin sulfate in addition to collagen, which all belongs to the natural moisturizing factor as important materials for maintaining skin moisture. Moreover it also has the role of preventing the conversion of tyrosine into melanin in the skin. Collagen, similar with the collagen structure in the skin and also the stratum corneum, which promotes the proliferation and repair of epithelial cell and replenishes amino acids and other nutrients, improves the living environment of skin cells, promotes the metabolism of skin and enhance circulation, fill and repair the damaged and aging skin, and also has a good compatibility, affinity and permeability with the skin. It can be fully absorbed by the skin and make the skin plump, stretch the wrinkles while being able to increase the density of the skin and resulting in tension. Therefore, collagen has a natural effect of moisturizing, whitening, eliminating freckle and wrinkle, and thus be widely used in the cosmetics industry. Currently on the market, many selling cosmetics such as masks, eye creams, skin creams contain collagen.
3. Application in Food Industry
There are two aspects of the application of collagen in food industry: its function and nutrition, respectively. Collagen peptides can be directly taken as a functional food alone, such as chewable tablets, and protein powders or enteral nutrition agents desired for athletes. Having foods rich in collagen can not only effectively delay the aging of body, strengthening the gluten and bone, enhancing physical fitness, but also lose the weight and blood pressure and have other health effects like replenishing calcium. Treating collagen with specific enzyme can break the intermolecular hydrogen bonds and further disrupting the original compact super-coiled structure. The resulting structure is more loosely-formed small molecules whose addition to the meat products can improve the connective tissue, increase the protein content, and have good taste and nutritious. The hydrolysis products of collagen can be used as frozen food modifier for using as thickening agent and have a low melting point, easy to be dissolved immediately after entrance into mouth. Therefore, collagen is widely used in jelly, sour cheese, ham, canned food, and bread. In addition, using the film-forming property can make collagen be the membrane materials for solidified and the labels of meat. Collagen can also be used as food adhesive for synthesizing film and as protective layer of food like meat and fish which has antioxidant activity while maintaining the bright color of meat.
4. Applications in other fields
With the continuous studies of collagen, there are increasing cases and promising prospects that collagen are applied in other fields. For example, extracting the collagen rich in the residual waste of tanning process can make collagen be the protein resources for feeding animal which realizes resource utilization. After the denaturation treatment of collagen, we can improve its textile properties and apply to the textile industry. In recent years, there are more and more cases that collagen has been applied to light industry such as paper industry, leather industry, and daily chemical industry. With the continuous development and progress of research techniques, it is clear that collagen will have broader application.
| [Chemical properties]
Yellowish flakes freeze-dried substance; It is the main component of skin, connective tissue, bone and teeth. Different sources of collagen have different types, but they all contain three α chain, and are arranged into three-helical conformation. The slight difference in primary structure results in the formation of different types of collagen. The denatured collagen is referred as gelatin.
| [Uses]
Surgical suture fibers; as the substrate for measuring collagen.
| [Synthetic method]
Use bovine tendon as the raw materials. Extract it with disodium hydrogen phosphate solution and KCl solution. Wash the residue, dehydrate and dry to obtain the finished product.
Type I collagen is the major protein component of the bone extracellular matrix, accounting for up to 90% of the organic matrix. Type I collagen synthesis follows translation in the endoplasmic reticulum of the pro-collagen alpha-1 (COL1α1) and alpha-2 (COL1α2) chains (see the Figure below).
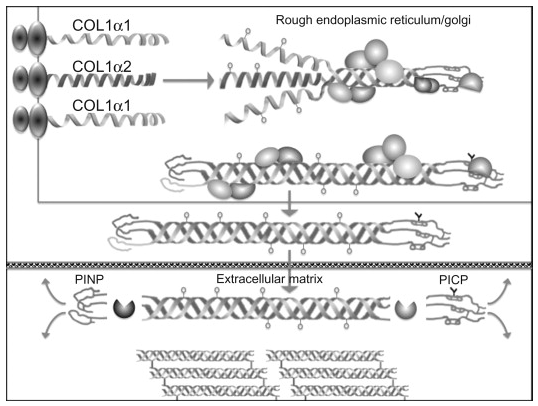 |
| Hazard Information | Back Directory | [Biochem/physiol Actions]
Collagen is essential for the mechanical integrity of tendons and bone. Rat tail tendon collagen is used in tissue engineering especially in the generation of 3-D scaffolds based gels. It has low antigenicity and is compatible with human gingival fibroblasts and human oral keratinocytes. |
|
|