2,3-Epoxy-3,7-dimethyloct-6-enol Chemische Eigenschaften,Einsatz,Produktion Methoden
Synthesis Reference(s)
Journal of the American Chemical Society, 95, p. 6136, 1973
DOI: 10.1021/ja00799a061Synthetic Communications, 14, p. 865, 1984
DOI: 10.1080/00397918408075730Tetrahedron Letters, 27, p. 707, 1986
DOI: 10.1016/S0040-4039(00)84079-4
Synthese
A mixture of activated 4 ? molecular sieves (1.80 g, 15?20 wt% based on geraniol) and CH2Cl2 (100 mL) was cooled to ?10 °C. L-(+)-Diethyl tartrate (1.00 g, 4.8 mmol), titanium(IV) isopropoxide (0.91 g, 3.2 mmol), and tert-butylhydroperoxide (19.4 mL, 97 mmol, 5.0 M in CH2Cl2) were added sequentially. After 10 min of stirring, the mixture was cooled to ?20 °C, and freshly distilled geraniol (10.0 g, 65 mmol, in 10 mL of CH2Cl2) was added dropwise over 15 min. After 45 min of stirring at ?20 to ?15 °C, the mixture was warmed to 0 °C. After an additional 5 min of stirring at 0 °C, the mixture was quenched sequentially with water (20 mL) and 4.5 mL of 30% aqueous NaOH saturated with solid NaCl. After 10 min of vigorous stirring, the reaction mixture was partitioned between CH2Cl2 and water. The combined organic extract was dried (MgSO4) and then fifiltered through Celite to give a clear colorless solution. Concentration followed by bulb to bulb distillation [bp (bath) = 100 °C at 0.1 mm Hg] gave the epoxide as a colorless oil (10.3 g, 91%).
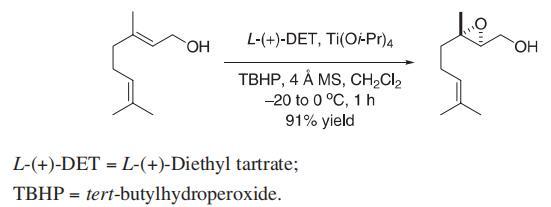
Reference: Taber, D. F.; Bui, G.; Chen, B. J. Org. Chem. 2001, 66, 3423?3426.
2,3-Epoxy-3,7-dimethyloct-6-enol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

