Fullerene C60 Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R36/37:Reizt die Augen und die Atmungsorgane.
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S22:Staub nicht einatmen.
Chemische Eigenschaften
black fine powder
Fullerene C60 is molecular carbon in the form of C60 and other members of the fullerene family were first synthesized in 1985. Solid C6o exhibits a large number of interesting physical and chemical properties. One of the theoretical predictions was that the C60 molecule itself would have a bulk modulus larger than that of diamond and the soft material under high pressure would become harder than diamond.
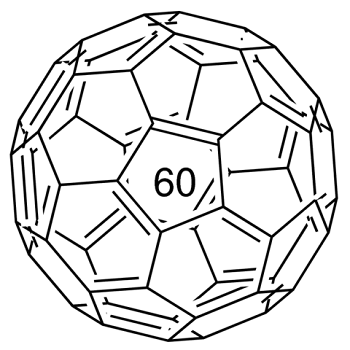
Fullerene-C60 (C60) is approximately 0.7 nm in diameter. It is a hollow, icosahedrally-shaped, closed-cage structure consisting of 60 sp2 hybridized carbon atoms which can be utilized for the preparation of novel carbon nanomaterials.
History
Fullerenes were first observed in 1985 in the sooty residue left after vaporizing carbon in a helium atmosphere. The discoverers thought the icosahedral structures with exactly 60 unsaturated carbon atoms resembled geodesic domes popularized by famous architect Buckminster Fuller and named them "buckminsterfullerenes" in his honor. The name has since been shortened to "fullerene," but they are sometimes also called "buckyballs."
Verwenden
fullerenes is also known as fullerines; buckyball. Fullerenes are soluble carbon molecules that are studied and incorporated into cosmetics for their anti-oxidant and free-radical scavenging properties. Some manufacturers cite significantly greater anti-oxidant potential than vitamins C and e and an ability to retain their antifree radical activity under a variety of external conditions such as heat, strong ultraviolet radiation. They are most commonly used to minimize potential reactions through an interaction with the immune system. Fullerenes are generally incorporated into antiaging and skin rejuvenation formations. The most common form of fullerenes is C60; other forms include C70, C76, and C84. They are the result of nanotechnology and its application in skin care.
Definition
An allotrope of carbon containing clusters of 60 carbon atoms bound in a highly symmetric polyhedral structure. The C60 polyhedron has a combination of pentagonal and hexagonal faces similar to the panels on a soccer ball. The molecule was named for the American architect Richard Buckminster Fuller (1895–1983) because its structure resembles a geodesic dome (invented by Fuller). The C60 polyhedra are informally called bucky balls. The original method of making the allotrope was to fire a high-power laser at a graphite target. This also produces less stable carbon clusters, such as C70. It can be produced more conveniently using an electric arc between graphite electrodes in an inert gas. The allotrope is soluble in benzene, from which it can be crystallized to give yellow crystals. This form of carbon is also known as fullerite.
The discovery of buckminsterfullerene led to a considerable amount of research into its properties and compounds. Particular interest has been shown in trapping metal ions inside the carbon cage to form enclosure compounds. Buckminsterfullerene itself is often simply called fullerene. The term also applies to derivatives of buckminsterfullerene and to similar cluster (e.g. C70). Carbon structures similar to that in C60 can also form small tubes, known as bucky tubes.
Allgemeine Beschreibung
Fullerite is a mixture of C
60 and C
70 fullerenes. It is a crystalline material with high light absorption and an energy band gap of 1.6 eV. It can be used as an acceptor molecule for the fabrication of bulk heterojunction polymeric solar cells.
Industrielle Verwendung
Buckyballs, the soccer-shaped molecules discoveredover a decade ago, are made entirelyof carbon atoms linked with unusual chemicalbonds.
Buckybowls, large fragments of buckyballs,have been synthesized. The moleculeshave the ability to take up electrons and givethem back later, under the right conditions, inhigher concentrations than buckyballs.The only practical application thus far hasbeen the computer industry’s use of anotherrelative, the buckytube, as an atomic-scaleprobe. Some researchers believe that the buckybowlsmay facilitate the development of plasticbatteries that would be lighter, smaller, andmore environmentally friendly than therechargeable batteries now used to power cellularphones and laptop computers.
The shape of the molecule may also allowit to bond with other molecules. It fits over thebuckyball much like a contact lens, and may beable to serve as a medium that links other substancesto the balls.
Fullerene C60 Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

