2-Aminoadenosine
|
|
|
- CAS-Nr.
- 2096-10-8
- Englisch Name:
- 2-Aminoadenosine
- Synonyma:
- 2-NH2-A;Aminoadenosine;2-AMINE ADENOSINE;2,6-DIAMINOPURINE RIBOSIDE;0/8/96;NSC 7363;2-NH2-Ar;2-NH2-rA;2-NH?-rA;2-AMinoadenosin
- CBNumber:
- CB2468756
- Summenformel:
- C10H14N6O4
- Molgewicht:
- 282.26
- MOL-Datei:
- 2096-10-8.mol
|
2-Aminoadenosine Eigenschaften
- Schmelzpunkt:
- 241-243°C (dec.)
- storage temp.
- Keep in dark place,Sealed in dry,Room Temperature
- Löslichkeit
- Aqueous Acid (Slightly, Heated, Sonicated), DMSO (Slightly, Sonicated), Ethanol
- Aggregatzustand
- Powder
- Siedepunkt:
- 798.5±70.0 °C(Predicted)
- Dichte
- 2.25±0.1 g/cm3(Predicted)
- pka
- 13.10±0.70(Predicted)
- Farbe
- White to Off-White
- InChIKey
- ZDTFMPXQUSBYRL-UUOKFMHZSA-N
- CAS Datenbank
- 2096-10-8(CAS DataBase Reference)
2-Aminoadenosine Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
2-Aminoadenosine is a known analogue of adenosine that engages in three hydrogen bonds of the W-C type, adding considerable stability to the double helix.
Chemische Eigenschaften
White Powder
Verwenden
2-Aminoadenosine is a nucleoside analog as inhibitor or substrate of adenosine kinase from M. tuberculosis. It is also used to synthesize 2′-O-methyl and 3′-O-methyl Guanosine.
Definition
ChEBI: 2-Aminoadenosine is a purine nucleoside.
Synthese
The synthesis of 2-aminoadenosine (2-AA): Assays were performed in 5 and 10% of aprotic dipolar co-solvents, at 60°C, twelve units/ml cell lysate were added to 150 ml of a solution kept thermostatically at 60 °C, and having the following composition of substrate solutions: 15mM Uridine/2 ' -Deoxyuridine,5 mM 2,6-Diaminopurine, and 30 mM potassium phosphate buffer, pH 7. After 5 hours, the reaction mixture was filtered by centrifugation at 2000 x g for 30 min, at 4°C, through an Amicon ultra-4 Centrifugal Filter Devices (Millipore, Bedford, MA) with a 3000-Da cutoff, and the filtrate was recovered.
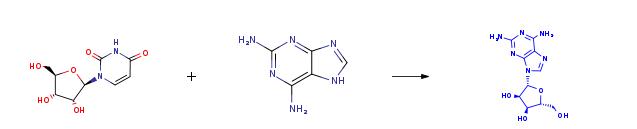
Einzelnachweise
Pyrazolo[3,4-d]pyrimidine ribonucleosides related to 2-aminoadenosine and isoguanosine: synthesis, deamination and tautomerism. DOI:
10.1039/B708736E An Efficient Process for Synthesis of 2′-O-methyl and 3′-O-methyl Guanosine from 2-aminoadenosine Using Diazomethane and the Catalyst Stannous Chloride. DOI:
10.1080/15257770500544529
2-Aminoadenosine Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
2-Aminoadenosine Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global( 365)Lieferanten
2096-10-8()Verwandte Suche:
- 2-AMINOADENOSINE
- 2-AMINOADENOSINE HPLC98+%
- (2R,3R,4S,5R)-2-(2,6-Diaminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
- 2-AMinoadenosin
- 2-NH2-Ar
- 2-Aminoadenosine≥ 99% (HPLC)
- 2,6-DiaMino-9-β-D-ribofuranosyl-9H-purine
- 2,6-DiaMinonebularine
- 2,6-DiaMinopurinosine
- 9-β-Ribosyl-2,6-diaMinopurine
- NSC 7363
- 2,6-Diamino-9-(beta-D-ribofuranosyl)purine
2,6-Diaminopurine Riboside
- Adenosine, 2-amino-
- Regadenoson Impurity 23
- 2, 6-Diaminopurine-riboside / 2-amino-adenosine
- 9H-Purine-2,6-diamine, 9-.β.-D-ribofuranosyl-
- 2-aminoadenine nucleoside
- 2-Aminoadenosine >
- 2,6-Diamino-9-(beta-D-ribofuranosyl)purine
- 2-Aminoadenosine USP/EP/BP
- TIANFU CHEM 2-Aminoadenosine
- 2-AMINE ADENOSINE
- 2,6-DIAMINOPURINE RIBOSIDE
- Aminoadenosine
- 2-NH2-A
- 2-NH2-rA
- 0/8/96
- (2R,3R,4S,5R)-2-(2,6-Diamino-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol
- 2-NH?-rA
- Regadenoson Impurity U18
- Regadson Impurity 18
- 2096-10-8
- 96-10-8
- 2090-10-8
- C10H14N6O4
- Amines
- Heterocycles
- Nucleotides and Nucleosides
- Nucleic acids
- Bases & Related Reagents
- Nucleotides
- 2096-10-8
- Nucleosides-Ribonucleosides

