5-Methoxybenzofuran-3(2H)-one
|
|
|
- CAS-Nr.
- 39581-55-0
- Englisch Name:
- 5-Methoxybenzofuran-3(2H)-one
- Synonyma:
- AKOS BC-0911;5-METHOXY-BENZOFURAN-3-ONE;5-Nitro-3-Benzofuranone (2);5-methoxy-1-benzofuran-3-one;5-methoxybenzofuran-3(2H)-one;5-Methoxy-3(2H)-benzofuranone;3(2H)-Benzofuranone, 5-Methoxy-;5-Methoxy-1-benzofuran-3(2H)-one;1H-1,4-Diazepine,hexahydro-4-(phenylmethyl)-
- CBNumber:
- CB3840863
- Summenformel:
- C9H8O3
- Molgewicht:
- 164.16
- MOL-Datei:
- 39581-55-0.mol
|
5-Methoxybenzofuran-3(2H)-one Eigenschaften
- storage temp.
- under inert gas (nitrogen or Argon) at 2-8°C
Sicherheit
- Risiko- und Sicherheitserklärung
- Gefahreninformationscode (GHS)
| Bildanzeige (GHS) |

|
| Alarmwort |
Warnung |
| Gefahrenhinweise |
| Code |
Gefahrenhinweise |
Gefahrenklasse |
Abteilung |
Alarmwort |
Symbol |
P-Code |
| H302 |
Gesundheitsschädlich bei Verschlucken. |
Akute Toxizität oral |
Kategorie 4 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P270, P301+P312, P330, P501 |
| H315 |
Verursacht Hautreizungen. |
Hautreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P302+P352, P321,P332+P313, P362 |
| H319 |
Verursacht schwere Augenreizung. |
Schwere Augenreizung |
Kategorie 2 |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
P264, P280, P305+P351+P338,P337+P313P |
| H335 |
Kann die Atemwege reizen. |
Spezifische Zielorgan-Toxizität (einmalige Exposition) |
Kategorie 3 (Atemwegsreizung) |
Warnung |
![GHS hazard pictograms]() src="/GHS07.jpg" width="20" height="20" /> src="/GHS07.jpg" width="20" height="20" /> |
|
|
| Sicherheit |
| P261 |
Einatmen von Staub vermeiden. |
| P305+P351+P338 |
BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen. |
|
5-Methoxybenzofuran-3(2H)-one Chemische Eigenschaften,Einsatz,Produktion Methoden
Synthese
5-Methoxybenzofuran-3(2H)-one is synthesised using 2-chloro-1-(2-hydroxy-5-methoxyphenyl)ethanone as raw material by chemical reaction. The specific synthesis steps are as follows:
To an ice-cooled solution of boron trichloride (1.2 equiv., 10 mmol, 10 ml of a solution of 1M BC13 in dichloromethane) under N2-atmosphere was added dropwise a solution of J (1 g, 8 mmol,) in dichloromethane (5 ml). Then, chloroacetonitrile (0.7 g, 10 mmol, 1.2 equiv. ) was added dropwise, followed by aluminum(III) chloride (0.5 g, 4 mmol, 0.5 equiv. ) in one portion. The reaction mixture was allowed to warm up to room temperature and was stirred for 6 h. The reaction mixture was diluted with dichloromethane and quenched with IN hydrochloric acid at 0°C. After stirring for 10 min, the aqueous layer was extracted with dichloromethane. The combined organic layers were washed with brine, dried (MgS04) and concentrated Purification by flash chromatography on silica gel (eluent: dichloromethane) afforded the desired product K (1 g, yield = 60percent). Intermediate K (0.15 g, 0.75 mmol, I equiv. ) and potassium acetate (0.22 g, 2.2 mmol, 3 equiv. ) were refluxed in ethanol (10 ml) for 1 h. After cooling, the reaction mixture was filtered and concentrated. The residue was mixed with water, and acidified with IN hydrochloric acid to pH = 1. The aqueous solution was extracted with ethyl acetate, the combined extracts were dried (MgS04), filtered and concentrated to afford the desired product L (110 mg, yield = 90 percent) as a pink solid The experimental procedures for the condensation reaction of compound L with 4-nitroaniline in acetic acid to form compound M, followed by Vilsmeier-Haack formulation and subsequent Knoevenagel condensation of the benzofuran carbaldehyde N with ethyl cyanoacetate to form compound 0, and finally, intramolecular cyclisation to compound 37 were performed using analogous procedures as exemplified in example 1 for the synthesis of compound f starting from compound a. Compound 37 was obtained as a yellow powder (30 mg, purity (LC) = 80 percent).
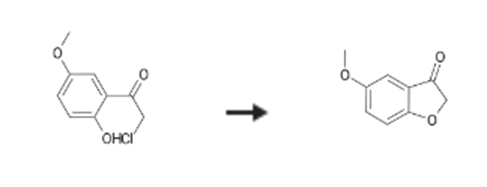
5-Methoxybenzofuran-3(2H)-one Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
5-Methoxybenzofuran-3(2H)-one Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global( 62)Lieferanten
- 5-METHOXY-BENZOFURAN-3-ONE
- AKOS BC-0911
- 5-Methoxy-1-benzofuran-3(2H)-one
- 3(2H)-Benzofuranone, 5-Methoxy-
- 5-Methoxy-3(2H)-benzofuranone
- 5-Nitro-3-Benzofuranone (2)
- 1H-1,4-Diazepine,hexahydro-4-(phenylmethyl)-
- 5-methoxybenzofuran-3(2H)-one
- 5-methoxy-1-benzofuran-3-one
- 39581-55-0

