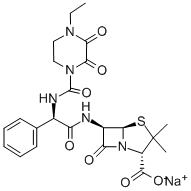
Piperacillin Sodium
- CAS No.
- 59703-84-3
- Chemical Name:
- Piperacillin Sodium
- Synonyms
- PIPERACILLIN SODIUM;t1220;pipril;C07361;cl227193;pentcillin;PIPERACILLIN NA;DBL Piperacillin;sodiumpiperacillin;PEPERACILLIN SODIUM
- CBNumber:
- CB00123429
- Molecular Formula:
- C23H26N5NaO7S
- Molecular Weight:
- 539.54
- MDL Number:
- MFCD00917471
- MOL File:
- 59703-84-3.mol
| Melting point | 183-185 C (dec.) |
|---|---|
| Boiling point | 793℃ |
| refractive index | 180 ° (C=0.8, H2O) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless to faintly yellow |
| form | Solid |
| color | White to Almost white |
| Water Solubility | Soluble in ethanol, water and methanol. |
| Merck | 14,7463 |
| BRN | 5373920 |
| Stability | Hygroscopic |
| InChIKey | WCMIIGXFCMNQDS-IDYPWDAWSA-M |
| CAS DataBase Reference | 59703-84-3(CAS DataBase Reference) |
| FDA UNII | M98T69Q7HP |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P280g-P284-P304+P340-P342+P311a-P501a |
| Hazard Codes | Xn |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37-45 |
| WGK Germany | 2 |
| RTECS | XI0180000 |
| HS Code | 29411099 |
| Toxicity | LD50 in mice, rats, dogs, monkeys (g/kg): 5, 2.7, >6, >4 i.v. (Takai) |
Piperacillin Sodium price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P8396 | Piperacillin sodium salt penicillin analog | 59703-84-3 | 1g | $106 | 2024-03-01 | Buy |
| Sigma-Aldrich | P8396 | Piperacillin sodium salt penicillin analog | 59703-84-3 | 5g | $428 | 2024-03-01 | Buy |
| TCI Chemical | P1774 | Piperacillin Sodium Salt >98.0%(N) | 59703-84-3 | 1g | $88 | 2024-03-01 | Buy |
| TCI Chemical | P1774 | Piperacillin Sodium Salt >98.0%(N) | 59703-84-3 | 5g | $321 | 2023-06-20 | Buy |
| Alfa Aesar | J66419 | Piperacillin sodium salt, 835-1007μg/mg | 59703-84-3 | 1g | $80.65 | 2024-03-01 | Buy |
Piperacillin Sodium Chemical Properties,Uses,Production
Description
Piperacillin sodium is a new beta-lactam antibiotic developed in 1977 by Ueo et al. at Toyama Chemical Company Ltd of Japan. It is licensed and marketed by Lederle Laboratories as Pipracil in the United States. The chemical name of piperacillin sodium is sodium [2S-[2α, 5α, 6β(S*)]]-6-[[[[(4-ethyl-2,3-dioxo1 -piperazinyl) carbonyll-amino] phenylacetyl] amino] -3,3-dimethyl-7-oxo-4-thia- 1 -azabicyclo[3.2.0]heptane-2-carboxylate. It was originally referred to as T1220. Piperacillin is an acylampicillin derivative and is similar in many respects to the previously marketed carboxypenicillins carbenicillin and ticarcillin, the two new ureidopenicillins mezlocillin and azlocillin, and the third-generation cephalosporin (-like) agents cefotaxime, moxalactam, and cefoperazone (the latter is not yet marketed and is structurally similar to piperacillin)[1].
Chemical Properties
When reconstituted, piperacillin sodium is a white to off-white hydroscopic cryodesiccated crystalline that gives a colorless to pale yellow solution. The empirical formula is C23H26N5NaO7S (1.85 mEq or 42.55 mg sodium per gram). One gram of piperacillin is soluble in 1.4 ml of water at 23°C, and the pH of the aqueous solution is 5.5-7.0.
Indications
Piperacillin has been approved for patients with severe infection caused by susceptible strains of specific organisms in the intra-abdominal, urinary tract, gynecologic, lower respiratory tract, skin and skin structure, bone and joint, gonococcal infections, and septicemia. As with other penicillins, piperacillin has a low frequency of toxicity.
benefits
Piperacillin sodium is a beta-lactam antibiotic with a broad range of antibacterial activity that includes gram-negative bacilli, gram-positive cocci (except penicillinase-producing S. aureus), and anaerobic pathogens such as Clostridium difficile, and Bacteroides fragilis. Piperacillin inhibits many of the members of the Enterobacteriaceae, including Klebsiella sp and Pseudomonas, at lower concentrations than required for carbenicillin and ticarcillin.
Metabolism
Piperacillin sodium is administered by intramuscular and intravenous injection and is widely distributed throughout body fluids and tissues. Like other newer penicillins, piperacillin is excreted by both renal and biliary mechanisms. The primary route of elimination is by glomerular filtration, which results in high urinary concentrations of the unchanged compound.
References
[1] C L Fortner, S C Schimpff, R S Finley. “Piperacillin sodium: antibacterial spectrum, pharmacokinetics, clinical efficacy, and adverse reactions.” Pharmacotherapy 2 6 (1982): 287–99.
Piperacillin Sodium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12459 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 990 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +86-86177 | admin@hbouhuang.com | China | 2952 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29897 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8826 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
View Lastest Price from Piperacillin Sodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-21 | Sodium tetraborate pentahydrate
12179-04-3
|
1kg | 99% | 100tons | Anhui Ruihan Technology Co., Ltd |
-

- Sodium tetraborate pentahydrate
12179-04-3
- 99%
- Anhui Ruihan Technology Co., Ltd
59703-84-3(Piperacillin Sodium)Related Search:
1of4