Deuterium Depleted Water
- CAS No.
- 7789-20-0
- Chemical Name:
- Deuterium Depleted Water
- Synonyms
- D2O;Deuterated water;WATER-D2;HEAVY WATER;heavywater-d2;water,heavy(d2-o);Heavy water, Water-d2;Ice-d;Ice-d2;Water-d
- CBNumber:
- CB00127904
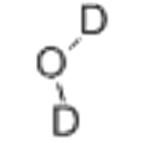
- Molecular Formula:
- D2O
- Molecular Weight:
- 20.03
- MDL Number:
- MFCD00044636
- MOL File:
- 7789-20-0.mol
- MSDS File:
- SDS
| Melting point | 3.8 °C(lit.) |
|---|---|
| Boiling point | 101.4 °C |
| Density | 1.107 g/mL at 25 °C |
| vapor pressure | 27.464hPa at 25℃ |
| refractive index |
n |
| Flash point | 101.4°C |
| storage temp. | Store below +30°C. |
| pka | pK (25°) 14.955 (molarity scale); 16.653 (mole fraction scale): A. K. Covington et al., J. Phys. Chem. 70, 3820 (1966) |
| form | Liquid |
| color | Colorless |
| Relative polarity | 0.991 |
| PH | 7 (H2O, 20℃) |
| Water Solubility | Miscible with water. |
| Sensitive | Moisture Sensitive |
| Merck | 14,2940 |
| Dielectric constant | 78.3(25℃) |
| Stability | Stable. Hygroscopic. |
| InChIKey | XLYOFNOQVPJJNP-ZSJDYOACSA-N |
| CAS DataBase Reference | 7789-20-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | J65BV539M3 |
| NCI Drug Dictionary | deuterium oxide |
| EPA Substance Registry System | Water-d2 (7789-20-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | ZC0230000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2845 10 00 | |||||||||
| NFPA 704 |
|
Deuterium Depleted Water price More Price(82)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 151882 | Deuterium oxide 99.9 atom % D | 7789-20-0 | 10x0.6ml | $51.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 151882 | Deuterium oxide 99.9 atom % D | 7789-20-0 | 10x0.75ml | $61.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.13366 | Deuterium oxide deuteration degree min. 99.9% for NMR spectroscopy MagniSolv? | 7789-20-0 | 10x0.75mL | $70.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.13366 | Deuterium oxide deuteration degree min. 99.9% for NMR spectroscopy MagniSolv? | 7789-20-0 | 10ml | $73.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.13366 | Deuterium oxide deuteration degree min. 99.9% for NMR spectroscopy MagniSolv? | 7789-20-0 | 25mL | $127 | 2024-03-01 | Buy |
Deuterium Depleted Water Chemical Properties,Uses,Production
Physical and chemical characteristics
Deuterium oxide, also known as "heavy water", "deuterium water", is the compound of oxygen and the heavy isotope of hydrogen, namely deuterium, which is the most important deuterium compound. It is called heavy water because its density is heavier than ordinary and its chemical formula is D2O.The liquid is colorless and odorless in normal temperature and pressure, containing the isotope of hydrogen with mass twice that of ordinary hydrogen. Compared to ordinary water, its chemical characteristic is relatively inactive with specific gravity of 1.10775 (25 ℃), melting point of 3.82 ℃, boiling point of 101.42 ℃. The content of heavy water in natural water is 1/5000. The ratio of deuterium to hydrogen in ordinary water is 1:6000 and the reserve of deuterium in Dead Sea or deep sea is relatively richer. There is no water origin in nature with rich deuterium. Heavy water is similar to ordinary water in appearance but with many different physical characteristics. The hydrogen bond strength and degree of association between heavy water molecules are both bigger than that of ordinary water molecules and the heavy water has higher melting point and boiling point. The vapor pressure of heavy water is smaller than that of ordinary water, which is the theoretical basis for enriching
Deuterium oxide using water distillation method. The viscosity of heavy water at 25℃ is 2.3% larger than that of ordinary water making the electrical conductivity of electrolyte in heavy water is smaller than in ordinary water and the specific inductive capacity of heavy water is smaller than ordinary water. The solubility of salts in heavy water is usually smaller and at 25 ℃ 1 g water can dissolve 0.3592 g sodium chloride while 1g heavy water can only dissolved 0.3592g sodium chloride. The distribution coefficient at 25℃ between carbon tetrachloride and water is 85:1 while 103:1 between carbon tetrachloride and deuterium oxide. The surface tension and ionic product ([D+7][OD+]=2×10-15) of heavy water are both smaller than that of water and in the same chemical reaction deuterium oxide reacts more slowly than water. Just like the concentrated sulfuric acid, heavy water can absorb water and must be kept in sealed containers. Heavy water can be used as nuclear moderator and heat reduction lubricant in the atomic reactor.
The information above is compiled by YaoYao in Chemicalbook.
Application history
In 1931, after finding Deuterium H C Urey also found an increase in centration of deuterium in liquid waste of electrolytic cell, proposing the idea that uses water electrolysis to enrich heavy water. In 1933 G N Louis electrolyze 10L liquid waste of electrolytic cell repeatly obtaining 0.5μL heavy water with concentration of about 65.7% and nearly pure deuterium oxide can be obtained after electrolysising the heavy water again. Some certain physical constants are obtained with this initial droplet heavy water. The relative molecular mass of heavy water is 20.0275, 11% larger than that of ordinary water of which the relative molecular mass is18.0153 and its physical characteristic is different from ordinary water. The melting point of heavy water is 3.82℃ with boiling point 101.42℃ and density (25℃) 1.10445 g/cm3. The neutron absorption cross section of heavy water is very small (only 5.3 x 10-4 barn) as the most ideal moderator in thermal reactor. Heavy water is used as moderator in many reactors such as Savannah River production reactor built in the United States in the 1950s, the power reactor used in nuclear power plants of Canada, and the research reactors in many countries. The deuterium in heavy water is an important part of fusion power reactor fuel. And deuterium oxide is extremely important strategic material. During world warⅡ, the Allies and the Nazi Germany attached great importance to heavy water production which was kept great secret. When the Allies learned that Nazi Germany was producing heavy water in the Norilsk factory of Norway, they first sent special detachment in 1943 to attack the factory and then bomb the factory using plane, making Nazi Germany suffered a serious setback in their efforts to develop nuclear weapons. In 1943 the United States built a number of large-scale deuterium oxide plants with annual production of about 20t. In the 90s many countries in the world can produce heavy water with annual production capacity of about 2000t.China started to develop heavy water production since the 50s and export deuterium oxide in the 80s. The price of heavy water per ton in the international market price is about $230000.
References: Dedi Chen, Zhou Li, and editor Guisheng Ku. National Defense Economy Dictionary. Beijing: Military Science Press. 2001.
Main use and function
Deuterium oxide can be used as neutron moderator and heat carrier in nuclear fission reactors and can also be used in chemical and biological research. The deuterium from heavy water electrolysis is the charging of hydrogen bombs.
Heavy water is mainly used as moderator in nuclear reactor to reduce the neutron velocity and control the nuclear fission process and also as coolant. Heavy water and deuterium are valuable tracer materials in the study of chemical and physiological changes. For example, diluted heavy water can run from more than ten meters to tens of meters per hour after irrigating trees with dilute water. The heavy water molecule can stay in human body for 14 days on average after measuring the content of deuterium in the urine of human drinking a large amount of diluted water. Deuterium can be used to research the digestion and metabolism of animal and plant instead of ordinary hydrogen. Concentrated or pure heavy water can not maintain the life of animals and plants and heavy water lead animal and plant to death at the concentration of 60%.
Marine deuterium oxide
Water consisting of deuterium and oxygen is indeed heavier than ordinary water because of deuterium in molecule and is called "heavy water" (D2O) . Heavy water is a kind of huge energy source and can be used as moderator and heat transfer medium in atomic energy reactor and also raw material of hydrogen bomb. The fusion reaction of deuterium can release huge energy. There is 200 tons of heavy water in seawater and once the heavy water is extracted the world's oceans can provide human beings with inexhaustible energy.
Now the method of producing heavy water in large scale including distillation, electrolysis, chemical exchange, adsorption and so on. Distillation based on the difference of vapor pressure of light water(H2O), semi-heavy water(HDO) and heavy water is one of the origin methods to separate deuterium oxide. Distillation was just applied in the first factory built in the United States to produce heavy water, which of course was later replaced by a more economical method. Chemical method is now more commonly used and also more economical and hydrogen sulfide (HDS)-water double-temperature exchange is one of the methods to extract heavy water from seawater. The exchange proceeds as the following reactions: H2O (liquid) + HDS(gas) =HDO(liquid)+H2S.At low temperature (25℃) the deuterium in hydrogen deuterium sulfide(HDS) transfers to liquid water forming HDO and at high temperature (100℃) deuterium in HDO transfers to H2S forming HDS. The deuterium is extracted from water after such process in which the ratio of D2O to H2S is 1:71600 and the amount of H2S is quite large but still much smaller than that of water in vapor method. Therefore the equipment used in such method is small and the cost is low. Generally speaking it is also commercial to concentrate heavy water primarily with such method. Hydrogen sulfide gas is toxic and corrosive but this method is better than others, which is currently the most widely used.

H2S-H2O double-temperature exchange process figure
Production method
Deuterium oxide resource is very rich and the content in seawater reaches 5 × 1014t. The purity of heavy water in reactor is required to reach 99.75% while the concentration of heavy water in natural water is very low with only 0.015% And the characteristics of heavy water production are large separation numbers, long balance time, large amount of material processing and energy consumption. The cost of heavy water production depends largely on that of initial enrichment and the chosen of concentration method from natural concentration to about 1% is very important. There are three main heavy water production methods as follows:
- Distillation method: using the vapor pressure characteristic of deuterium compounds to enrich deuterium. The main raw materials are hydrogen, ammonia, water and so on. The distillation factor of liquid hydrogen is large but the low temperature technology and equipment limit the scale of production. Water distillation is easy and reliable to operate but the separation coefficient is too small with large energy consumption. The separation coefficient of ammonia distillation is slightly larger than that of water and the latent heat is small. But the limited ammonia source makes it uneconomical to be used for initial enrichment.
- Electrolysis method: the electrolysis separation coefficient of deuterium is about 10. It is the main method producing deuterium oxide before the 1950’s but cannot be used singly due to large energy consumption.
- Chemical exchange method: as the the most economical way now to produce heavy water, the actual process is divided into single-temperature and double-temperature exchange method. And the double-temperature exchange process using hydrogen sulfide and water is nowadays the main method to produce low-concentration heavy water in industrial scale. In addition there are other methods still in development such as hydrogen-adsorption alloy adsorption-separation method and laser separation method.
Chemical Properties
colourless liquid
Uses
Deuterium Oxide is used to prepare specifically labelled isotopologs of organic compounds.
Uses
Deuterium oxide is used in nuclear magnetic resonance spectroscopy (NMR). It is also useful in the identification of labile hydrogens. As a source of deuterium, it is utilized for preparing specifically labeled isotopologs of organic compounds. It is often used as a substitute for water in the analysis of proteins in solution by using fourier transform infrared spectroscopy (FTIR). It finds application in certain types of nuclear reactors and in tritium production.
Uses
To study chemical reaction rates and mechanisms. The cross section of deuterium for the capture of thermal neutrons is very low which makes it useful, in the form of heavy water, as a neutron moderator in nuclear reactors. Produces a considerable decrease in neutron energy per collision.
Definition
deuterium oxide: Water in which hydrogen atoms, 1H,are replaced by the heavier isotopedeuterium, 2H (symbol D). It is acolourless liquid, which forms hexagonalcrystals on freezing. Its physicalproperties differ from those of ‘normal’water; r.d. 1.105; m.p. 3.8°C; b.p.101.4°C. Deuterium oxide, D2O, occursto a small extent (about 0.003%by weight) in natural water, fromwhich it can be separated by fractionaldistillation or by electrolysis. Itis useful in the nuclear industry becauseof its ability to reduce the energiesof fast neutrons to thermalenergies and because its absorptioncross-section is lower than that of hydrogenand consequently it does notappreciably reduce the neutron flux.In the laboratory it is used for labellingother molecules for studies ofreaction mechanisms. Water alsocontains the compound HDO.
General Description
Deuterium oxide (D2O) is a 100% isotopically enriched NMR (Nuclear Magnetic Resonance) solvent. It is widely employed in high resolution NMR studies. Various thermodynamic properties (such as intermolecular vibrational frequencies, energy of the hydrogen bond, free energy, enthalpy and entropy) of liquid deuterium oxide have been evaluated. Ionization constant for D2O (in the range of 5-50°C), pK values (at 25°C) and enthalpy, entropy, heat capacity change (for the dissociation of D2O) have been reported.
Purification Methods
Distil it from alkaline KMnO4 [de Giovanni & Zamenhof Biochem J 92 79 1963]. NOTE that D2O invariably contains tritiated water and will therefore be RADIOACTIVE; always check the radioactivity level of D2O in a scintillation counter before using.
Deuterium Depleted Water Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Auschemicals Pty Ltd | +61406202619 | info@auschemicals.com | Australia | 426 | 58 |
| Yurui (Shanghai) Chemical Co., Ltd. | +86-021-50456736 +8613761615711 | yuki@riyngroup.com | China | 1028 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20288 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Accela ChemBio Inc. | +1-858-6993322 | info@accelachem.com | United States | 19943 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5600 | 58 |
Related articles
- Deuterium Oxide: From Nuclear Power Advancements to Therapeutic Potential in Health Research
- Deuterium oxide(D2O) is a form of water in which hydrogen atoms are all deuterium rather than the common hydrogen-1 isotope (1....
- Oct 22,2024
- Deuterium oxide: Chemical structures, Reactions and Applications
- Deuterium oxide (D2O), also known as "heavy water", is a colourless liquid.
- May 11,2023
View Lastest Price from Deuterium Depleted Water manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-12-24 | DEUTERIUM OXIDE
7789-20-0
|
US $100.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2021-07-10 | DEUTERIUM OXIDE
7789-20-0
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2020-01-13 | DEUTERIUM
7782-39-0
|
US $6.68 / KG | 1KG | 97%-99% | 1kg-1000kg | Career Henan Chemical Co |
-

- DEUTERIUM OXIDE
7789-20-0
- US $100.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- DEUTERIUM OXIDE
7789-20-0
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- DEUTERIUM
7782-39-0
- US $6.68 / KG
- 97%-99%
- Career Henan Chemical Co





