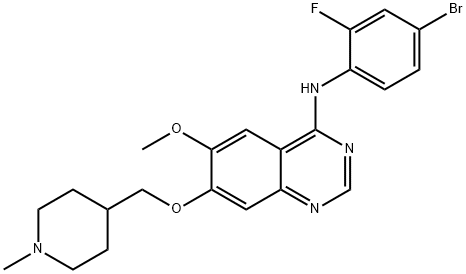
Vandetanib
- CAS No.
- 443913-73-3
- Chemical Name:
- Vandetanib
- Synonyms
- CS-97;D6474;Zactima;ZD 6474;AZD6474;Vetanib;VANDETANIB;Vandetanib INN;Vandetanib base;ZD6474; ZACTIMA
- CBNumber:
- CB01011762
- Molecular Formula:
- C22H24BrFN4O2
- Molecular Weight:
- 475.35
- MDL Number:
- MFCD07772346
- MOL File:
- 443913-73-3.mol
| Melting point | 240-2430C |
|---|---|
| Boiling point | 538.2±50.0 °C(Predicted) |
| Density | 1.406 |
| Flash point | 279.3℃ |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (30 mg/ml); Ethanol (10 mg/ml with warming) |
| pka | 8.92±0.10(Predicted) |
| form | solid |
| color | White to off-white |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| InChIKey | UHTHHESEBZOYNR-UHFFFAOYSA-N |
| SMILES | N1=C2C(C=C(OC)C(OCC3CCN(C)CC3)=C2)=C(NC2=CC=C(Br)C=C2F)N=C1 |
| CAS DataBase Reference | 443913-73-3 |
| FDA UNII | YO460OQ37K |
| NCI Dictionary of Cancer Terms | vandetanib; ZD6474 |
| NCI Drug Dictionary | vandetanib |
| ATC code | L01EX04 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H312-H315-H318-H332-H335-H227-H410 | |||||||||
| Precautionary statements | P210-P261-P264-P270-P271-P280-P302+P352-P304+P340-P305+P351+P338-P310-P330-P332+P313-P362-P370+P378-P403+P233-P403+P235-P405-P501-P273 | |||||||||
| Safety Statements | 24/25 | |||||||||
| HS Code | 29333220 | |||||||||
| Hazardous Substances Data | 443913-73-3(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Vandetanib price More Price(51)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 14706 | Vandetanib ≥98% | 443913-73-3 | 5mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 14706 | Vandetanib ≥98% | 443913-73-3 | 10mg | $65 | 2024-03-01 | Buy |
| Cayman Chemical | 14706 | Vandetanib ≥98% | 443913-73-3 | 25mg | $108 | 2024-03-01 | Buy |
| Cayman Chemical | 14706 | Vandetanib ≥98% | 443913-73-3 | 100mg | $310 | 2024-03-01 | Buy |
| Usbiological | 291899 | Vandetanib | 443913-73-3 | 100mg | $319 | 2021-12-16 | Buy |
Vandetanib Chemical Properties,Uses,Production
Anticancer drugs
Vandetanib is a kind of small molecule multi-targeted tyrosine kinase inhibitor studied and developed by the British AstraZeneca Company. In April 2011, it was approved the US FDA for entering into market under the trade name Zactima. The drug, as a tablet, can be applied to the treatment of advanced medullary thyroid cancer of adult patients.
Vandetanib is a multi-targeted tyrosine kinase inhibitor, and belongs to the Anilinoquinazoline compounds, called "second generation Iressa”. It not only acts on the tumor cells, EGFR, VEGFR and RET tyrosine kinases, but can also inhibit other kind of tyrosine kinases and serine/threonine kinases. Vandetanib is the first approved drugs approved for treatment of medullary thyroid carcinoma. It is suitable for treating unresectable, locally advanced or metastatic-symptoms or progressive medullary thyroid carcinoma. A randomized, placebo-controlled clinical trial results have showed that vandetanib can significantly delay the progression time of locally advanced or metastatic medullary thyroid cancer. The recommended daily dose is 300 mg (oral), when the patient exhibits tolerance to drugs or being not able to tolerate the toxicity, they should stop treatment immediately. Those most common adverse reactions of this medicine include diarrhea, rash, acne, nausea, hypertension, headache, fatigue, loss of appetite and abdominal pain.
The adverse reactions is dose-related; at <300 mg/d, the patient has a well tolerance with the maximum tolerated dose (MTD) being 300mg. There are many kinds disease types contained in the Ⅱ phase clinical study. The NSCLC clinical trials of vandetanib are currently under way for China.
British AstraZeneca Company, in May 2001 and October 2001, respectively, had obtained preferential access to the world's patent (WO2001032651, WO 2001074360) and applied corresponding protection on the formula, synthesis methods and pharmaceutical compositions of these compounds (including bonus salt). Vandetanib can inhibit the development of medullary thyroid carcinoma, and is the first FDA-approved drug for the treatment of the disease and will provide support for the treatment of advanced medullary thyroid cancer in adult patients.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Treatment of advanced (non-small cell lung cancer) NSCLC
A study published in the [Journal of Clinical Oncology] have showed that compared with gefitinib which only has inhibitory effect on EGFR, vandetanib can effectively extend the progression-free survival period of the non-small cell lung cancer (NSCLC) patients.
In this phase II clinical trial conducted by doctors Ronald B. Natale from Los Angeles Cedars-Sinai Cancer Center, clinical study compared the treatment efficacy of vandetanib (300 mg/d) and gefitinib (250mg/d) on 168 cases of NSCLC patients who had been subject to failing first-line or second-line chemotherapy. Compared with gefitinib, vandetanib significantly increased efficiency and prolonged the progression-free survival, period respectively, by 8% and 1%, 11.9 weeks and 8.1 weeks, (P = 0.011). In clinical trials, if there is progression of the disease or if the patient can’t tolerate the toxicity, they allowed the patients to change the treatment regimen. Experimental results had shown that using gefitinib for replacing vandetanib in patients gave a disease control rate of 14% while using vandetanib to replace gefitinib gave a disease control rate of 32%. The overall survival period in the case of vandetanib → gefitinib was 6.1 months while it was 7.4 months in the cases of gefitinib → vandetanib.
Preparation method
Use 4-hydroxy-3-methoxy-benzoic acid ethyl ester as a starting material, go through substitution, reduction, nitrification, reduction, cyclization aromatization to give 4-chloro-6-methoxy--7-(N-methyl piperidin-4-ylmethoxy) quinazoline (A), A was then reacted with 4-bromo-2-aniline to obtain the targeted compound vandetanib.
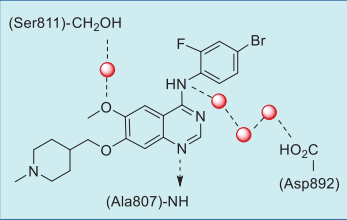
Binding Mode
Vandetanib is anchored to the hinge of RET by
forming a critical hydrogen bond between the N1
nitrogen of the quinazoline group and the amide NH
of Ala807 of the hinge (Figs. 2, 3). The methoxy
oxygen interacts with the side chain hydroxyl of
Ser811 via a bound water molecule, while the C4 NH
is involved in a network of hydrogen bonds with three
water molecules and the side chain carboxylate of Asp892.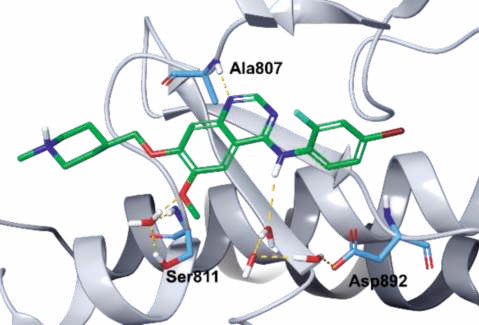
Description
In April 2011, the U.S. FDA approved vandetanib (ZD6474) for the treatment of symptomatic or progressive medullary thyroid cancer (MTC) in adult patients with inoperable advanced ormetastatic disease. Vandetanib inhibits KDR/VEGFR2, VEGFR3, EGFR, and RET kinases with IC50's of 40, 110, 500, and <100 nM, respectively. In athymic mice bearing MTC tumors, a 14.5-fold reduction of tumor volume was observed after 45 days of treatment with vandetanib at 50 mg/kg/day. The decrease in tumor volume was accompanied by decreases in mitotic index (Ki67) and tumor angiogenesis in treated xenografts. Key steps in the synthesis of vandetanib include the displacement of the chlorine atom from 7-benzyloxy-4-chloro-6-methoxyquinazoline with 4-bromo-2- fluoroaniline under acidic conditions in a protic solvent and a Mitsunobu reaction of a N-protected piperidine alcohol with a phenol.
Chemical Properties
Yellow Solid
Originator
Astra Zeneca (United Kingdom)
Uses
Vandetanib is a broad spectrum, orally available kinase inhibitor that targets primarily tyrosine kinases, including vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR), with IC50 values in the nanomolar range. It also potently blocks non-receptor tyrosine kinases, including ABL, RET, and SRC, as well as several serine/threonine kinases. Primarily because of its effects on receptor tyrosine kinases like VEGFR and EGFR, vandetanib inhibits angiogenesis, cell growth, and metastasis and is effective against certain forms of cancer.[Cayman Chemical]
Uses
Vandetanib (Zactima, ZD6474) is a potent inhibitor of VEGFR2 with IC50 of 40 nM.
Uses
Vandetanib (ZD6474) is a potent inhibitor of VEGFR2 with IC50 of 40 nM
Uses
Vandetanib is a once-daily oral inhibitor of vascular endothelial growth factor receptor-2 and epidermal growth factor receptor kinase activity. The activity of Vandetanib plus Docetaxel was assessed in patients with previously treated non-small-cell lung cancer (NSCLC).
Definition
ChEBI: Vandetanib is a quinazoline that is 7-[(1-methylpiperidin-4-yl)methoxy]quinazoline bearing additional methoxy and 4-bromo-2-fluorophenylamino substituents at positions 6 and 4 respectively. Used for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease. It has a role as a tyrosine kinase inhibitor and an antineoplastic agent. It is an aromatic ether, a secondary amine, a member of quinazolines, a member of piperidines, an organobromine compound and an organofluorine compound.
brand name
Zactima (AstraZeneca);Caprelsa.
General Description
Class: receptor tyrosine kinase; Treatment: RET-altered thyroid cancers; Other name: AZD-6474; Elimination half-life = 19 days; Protein binding = 90%
Clinical Use
Vandetanib, an oral VEGF, EGF, and RET receptor tyrosine kinase inhibitor, was developed by AstraZeneca for the treatment of symptomatic or aggressive medullary thyroid cancer (MTC) in patients with advanced or metastatic disease. This is the first drug approved for the treatment of MTC. Trials for other cancer indications such as small-cell lung cancer (SCLC), breast cancer, head and neck cancer, colorectal cancer, hormone-resistant prostate cancer, and papillary thyroid cancer are currently being explored. While AstraZeneca had previously developed ZD-4190 which displays similar efficacy and pharmacokinetic profile to vandetanib, vandetanib exhibited significantly improved solubility.
Synthesis
Vandetanib contains a 4-anilinoquinazoline scaffold similar to other EGFR inhibitors, and the synthesis described below is based on a recent patent (Scheme).
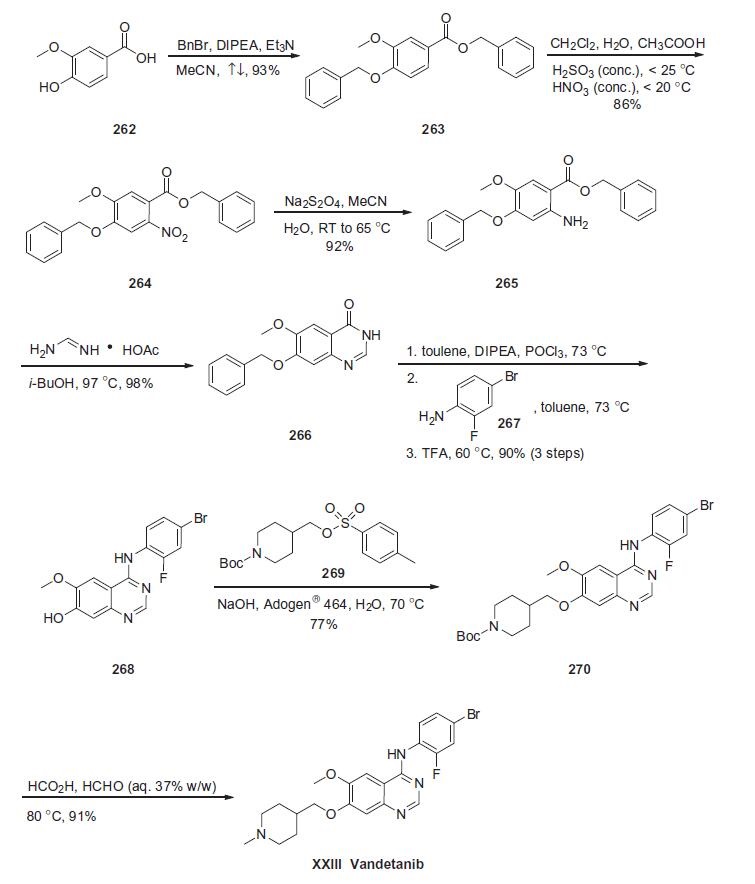
Commercially available vanillic acid (262) was treated with benzyl bromide, DIPEA and Et3N to give ethereal ester 263 in 93% yield. Arene 263 was then subjected to nitration conditions to provide nitroarene 264 in 86% yield, which underwent immediate reduction with sodium dithionite in acetonitrile and water to give aniline 265 in 92% yield. Aniline 265 was then treated with foramidine acetate in isobutanol which affected an intramolecular cyclization reaction, giving rise to dihydroquinazolin-4-one 266 in 98% yield. Heterocycle 266 was treated with phosphorous oxychloride and the resulting quinazoline chloride was subsequently reacted with 4-bromo-2-fluoroaniline 267 and trifluoroacetic acid to give hydroxyaniline 268 in 90% for the three-step sequence. Phenolic azacycle 268 was then alkylated with sulfonate 269 to furnish piperidine 270 in 77% yield. Subsequent treatment with formic acid and aqueous formaldehyde under elevated temperatures gave vandetanib (XXIII) in 91% yield.
target
VEGFR2/RET
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: possibly increased risk of ventricular
arrhythmias with methadone - avoid.
Anti-arrhythmics: possibly increased risk of
ventricular arrhythmias with amiodarone or
disopyramide - avoid.
Antibacterials: possibly increased risk of ventricular
arrhythmias with parenteral erythromycin and
moxifloxacin - avoid; concentration reduced by
rifampicin - avoid.
Antihistamines: possibly increased risk of ventricular
arrhythmias with mizolastine - avoid.
Antimalarials: possibly increased risk of ventricular
arrhythmias with artemether with lumefantrine -
avoid.
Antipsychotics: possibly increased risk of ventricular
arrhythmias with amisulpiride, chlorpromazine,
haloperidol, pimozide, sulpiride and zuclopenthixol
- avoid; avoid concomitant use with clozapine, risk
of agranulocytosis.
Beta-blockers: possibly increased risk of ventricular
arrhythmias with sotalol - avoid.
Cytotoxics: possibly increased risk of ventricular
arrhythmias with arsenic trioxide - avoid.
Hormone antagonist: possibly increased risk of
ventricular arrhythmias with toremifene - avoid.
5HT3
-receptor antagonists: possibly increased risk of
ventricular arrhythmias with ondansetron - avoid.
Pentamidine: possibly increased risk of ventricular
arrhythmias - avoid.
IC 50
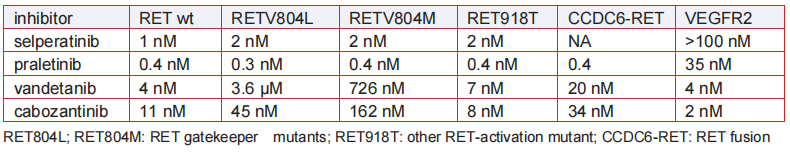
Metabolism
N-desmethyl-vandetanib is primarily produced by
CYP3A4, and vandetanib-N-oxide is primarily produced
by flavin-containing monooxygenase enzymes FMO1
and FMO3.
Unchanged vandentanib and metabolites vandetanib
N-oxide and N-desmethyl vandetanib were detected in
plasma, urine (25
%) and faeces (44
%).
storage
Store at -20°C
References
Wedge et al. (2002), ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis and tumor growth following oral administration; Cancer Res., 62 4645 Ciardiello et al. (2003), Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase; Clin. Cancer Res., 9 1546 Herbst et al. (2007), Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis; Expert Opin. Investig. Drugs, 16 239
Vandetanib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TianRui Pharmaceutical Chemical Co., Ltd. | +86-0551-68665055 +86-+86-18616906106 | sales@trywchem.com | China | 148 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 485 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 | allison@yan-xi.com | China | 5854 | 58 |
| Yangzhou Qinyuan Pharmatech Co.,ltd | +86-18752526868 | jennysun@yzqyyykj.com | China | 81 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20237 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 | sales@fine-chemtech.com | CHINA | 885 | 55 |
Related articles
- What to know when taking Vandetanib?
- Vandetanib works by blocking the action of abnormal proteins that signal cancer cells to multiply, thereby helping to slow or ....
- Mar 24,2025
- Vandetanib- Application in Cancer Therapy
- Vandetanib is a multiple signal transduction inhibitor including the RET tyrosine kinase, epidermal growth factor receptor (EG....
- Dec 10,2019
View Lastest Price from Vandetanib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-18 | Vandetanib
443913-73-3
|
US $0.00-0.00 / KG | 1KG | 99% | 20TONS | Sinoway Industrial co., ltd. | |
 |
2025-04-16 | Vandetanib
443913-73-3
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-03-07 | Vandetanib
443913-73-3
|
US $40.00 / kg | 1kg | 0.99 | 10 tons | Hebei Yanxi Chemical Co., Ltd. |
-

- Vandetanib
443913-73-3
- US $0.00-0.00 / KG
- 99%
- Sinoway Industrial co., ltd.
-

- Vandetanib
443913-73-3
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Vandetanib
443913-73-3
- US $40.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.