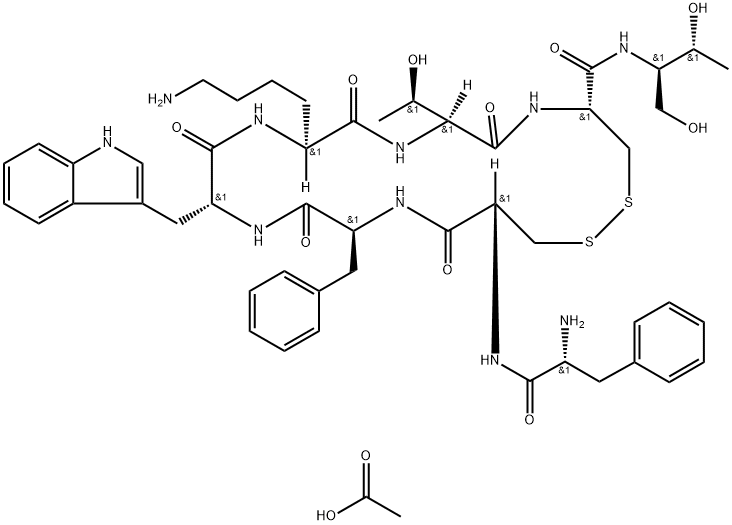
Octreotide
- CAS No.
- 79517-01-4
- Chemical Name:
- Octreotide
- Synonyms
- SANDOSTATIN;(D-Phe)-Cys-Phe-(D-Trp)-Lys-Thr-Cys-Thr-ol (Disulfide bond 2-7);Ktreotide;800016R-1;OCTREOTIDE;SMS-201-995;SMS 201-995ac;Octreotide/OTC;Sandostatin LAR;Octreotide xAcetate
- CBNumber:
- CB0279552
- Molecular Formula:
- C51H70N10O12S2
- Molecular Weight:
- 1079.3
- MDL Number:
- MFCD08277638
- MOL File:
- 79517-01-4.mol
- MSDS File:
- SDS
| Melting point | 153-156°C |
|---|---|
| alpha | D20 -42° (c = 0.5 in 95% acetic acid) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: soluble |
| form | Solid |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | XQEJFZYLWPSJOV-XJQYZYIXSA-N |
| CAS DataBase Reference | 79517-01-4 |
| NCI Dictionary of Cancer Terms | octreotide |
| FDA UNII | 75R0U2568I |
| NCI Drug Dictionary | Sandostatin |
| ATC code | H01CB02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P305+P351+P338 |
| WGK Germany | 3 |
| HS Code | 2937190000 |
| Toxicity | TDLo scu-man: 2587 mg/kg:GIT,LVR LANCAO348,1668,1996 |
Octreotide price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR1880 | Octreotide Acetate Pharmaceutical Secondary Standard; Certified Reference Material | 79517-01-4 | 10mg | $342 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1477604 | Octreotide acetate | 79517-01-4 | 4.37mg | $1570 | 2024-03-01 | Buy |
| Tocris | 1818 | Octreotide | 79517-01-4 | 1 | $281 | 2021-12-16 | Buy |
| TRC | O239950 | Octreotide Acetate | 79517-01-4 | 25mg | $175 | 2021-12-16 | Buy |
| ChemScene | CS-0944 | Octreotide Acetate 99.83% | 79517-01-4 | 10mg | $60 | 2021-12-16 | Buy |
Octreotide Chemical Properties,Uses,Production
Digestive system drugs
Octreotide belongs to digestive system drugs, which is an artificially synthetic cyclic octapeptide compound, and is a homolog of natural somatostatin. It contains the pharmacological activity of a natural somatostatin, and has long-lasting effects (the half-lifetime of this product is 1.5-hour, while it is 2 to 3 minutes of natural somatostatin). The product selectively inhibited the secretion of growth hormone, glucagon and insulin, and the effect is stronger than natural. It is proved by animal experiments: intravenous this product for 15 minutes, the inhibition of growth hormone release is 70 times higher than the natural somatostatin, and the inhibition of insulin release is 3 times than the natural somatostatin. It has also the inhibition effect to thyrotropin, adrenocorticotropic hormone, gastrin, cholecystokinin, vasoactive intestinal polypeptide. As well as it can inhibit the secretion of gastric acid, pancreatic amylase, lipase and pepsin. It can slow down pass through time of gastrointestinal tract, which promotes the absorption of water and electrolytes. It has the contract effect of visceral blood vessels, which can reduce splanchnic blood flow, and reduce the blood flow of liver. Thus, it reduces the portal pressure and portal blood flow, and reduces excessive secretion of intestinal tract, and it may enhance the intestinal absorption of water and the Na+.
The amino acids of 1,4,8 positon is unnatural amino acids on the chemical structure of the product, so it is less susceptible to enzyme hydrolysis, and it has more longer half-lifetime. It is absorbed rapidly and completely by subcutaneous injection of the product. The time to peak plasma concentration is 0.5-hour, and the volume of distribution is 0.27 L/kg. The half-lifetime is 1.5-hour, and the metabolic clearance rate is 160 ml/min. It is biphasic elimination as intravenous injection of this product, the half-lifetime of α phase and β phase are 10 minutes and 90 minutes, respectively. The plasma protein binding rate is 65%.
Octreotide is clinically used for the treatment of esophageal varices caused by portal hypertension, acromegaly, gastrointestinal bleeding including cirrhosis, esophageal varices bleeding, peptic ulcer bleeding and stress ulcer bleeding, pancreatic diseases including heavy acute pancreatitis, pancreatic trauma, or pancreatic fistula after surgery, and prevention of complications after pancreas surgery. It is also used for gastrointestinal fistula, digestive endocrine tumors such as vasoactive intestinal peptide tumors, gastrinoma, glucagon tumors, ectopic ACTH syndrome, carcinoid syndrome, and it is used for acromegaly, exophthalmus hyperthyroidism disease, after the removal of the entire colon appearing persistent and intractable diarrhea, AIDS-related diarrhea. Intrathecal injection of this product can treat cancer pain.
Dosage and administration
1. prevention of complications of pancreatic surgery: one hour before surgery, subcutaneous injection of 0.1mg octreotide; then 0.1mg subcutaneously 3 times a day for seven days.
2. treatment of varices caused by esophagus portal hypertension: 0.1mg intravenous injection, then 0.5mg, every two hours by intravenous drip.
3. stress ulcers and gastrointestinal bleeding: 0.1mg subcutaneously 3 times a day.
4. severe pancreatitis: 0.1mg subcutaneous injection, 4 times a day for 3 to 7 days; pancreatic injury or pancreatic fistula after surgery, subcutaneous injection, each 0.1mg, every 8 hours for 7 to 14 days.
5. gastrointestinal fistula and digestive tract endocrine system of cancer adjuvant therapy: subcutaneous 0.1mg, 3 times a day, a course for10 to 14 days.
6. digestive system endocrine tumors, Graves' disease, acromegaly and AIDS-related diarrhea: subcutaneous 0.1mg, 3 times a day, acromegaly treatment for 10 to 14 days. There are injection site pain or tingling, and it is usually ease after 15 minutes.
Gastrointestinal adverse reactions include anorexia, nausea, vomiting, diarrhea, abdominal cramps. Occasionally it will appear hyperglycemia, cholelithiasis, abnormal glucose tolerance, and liver function abnormalities (γ-glutamyl transferase increased and transaminase slightly increased) and others. Patients with dysfunction of kidney and pancreatic, and cholelithiasis should be used with caution. Pregnant women, lactating women and children are prohibition.
The above information is edited by the chemicalbook of Kui Ming.
Category
toxic substances
Toxicity grading
highly toxic
Acute toxicity
intravenous-rat LD50: 18.1 mg/kg; intravenous-mouse LD50: 72.3 mg/kg
Flammability hazard characteristics
combustible; fire decomposition of toxic nitrogen oxide compounds, sulfur oxide fumes
storage properties
cold warehouse, ventilated and dry; and separately stored with food raw materials
Extinguishing media
water, carbon dioxide, dry power, sandy soil
Chemical Properties
Colourless to off-white lyophilised solid
Originator
Sandostatin,Novartis Pharma AG,Switz.
Uses
Octreotide acetate USP (Sandostatin) is used to treat Mestastatic carcinoid tumors; vasoactive intestinal peptide-secretory tumors.
Uses
natural hormone somatostatin
Uses
Octapeptide analog of Somatostatin. A gastric antisecretory agent. Used in treatment of acromegaly
Indications
Octreotide is useful in inhibiting the secretion of various autacoids and peptide hormones by metastatic carcinoid tumors (serotonin) and islet cell carcinomas of the pancreas (gastrin, glucagon, insulin, vasoactive intestinal peptide). The diarrhea and flushing associated with the carcinoid syndrome are improved in 70 to 80% of the patients treated with octreotide. Its side effects, which are usually mild, include nausea and pain at the injection site. Mild transient hypoglycemia or hyperglycemia may result from alterations in insulin, glucagon, or growth hormone secretion.
Indications
Octreotide (Sandostatin) is a synthetic somatostatin analogue. It is used in a variety of situations and must be given subcutaneously or intravenously. Most commonly, it is used as a continuous intravenous infusion in patients hospitalized with bleeding varices, because it decreases splanchnic circulation and therefore reduces portal pressures.A long-acting depot form is approved for the suppression of severe diarrhea and flushing associated with malignant carcinoid syndrome and for the treatment of the profuse watery diarrhea associated with vasoactive intestinal peptide tumor.
Manufacturing Process
Synthesis of octreotide and derivative thereof can be carried out by two methods. The first method is synthesized initially by fragment condensation solution phase procedures. The synthetic process of octreotide has been described by Bauer et al. (1). The second method is the synthese by solidphase procedures. Edward et al. (2) isolated side chain protected octreotide with a total yield of 14% by cleaving the protected peptide from the resin with threoninol. Arano et al. (3) carried out another solid phase method for octreotide. and produced it in overall 31.8% yield based on the starting FmocThr(tBu)-ol-resin. The basic difference from the other procedures already described is that the introduction of the threoninol is carried out upon the protected peptidic structure (resin-free), which, when appropriately activated, leads quantitatively and without needing to make temporary protections upon the threoninol, to the protected precursor of octreotide, which in turn, with a simple acid treatment leads to octreotide with very high yields.
At first the Fmoc-Cys-Cl-trityl-resin was prepared. The incorporation of the Fmoc-Cys(trt)-OH residue upon 2Cl-Trt resin is accomplished with an excess of 1 eq. of Fmoc-Cys(Trt) and 2.5 eq. of N,N'-diisopropylethylamine (DIEA).
2.93 g (5.0 mmol) of Fmoc-Cys(Trt) are incorporated upon 5 g of resin (f = 1.28 mmol/g of resin, 6.4 mmol). The resin and the amino acid are weighed in separate containers and left to dry in a vacuum with KOH, for a minimum of two hours. A 1/1 solution of DIEA and CH2Cl2 (DCM) (dry on a 4A sieve) is
prepared. The already dry amino acid is dissolved with dry DCM at a concentration of 0.1 g of resin per ml, adding the minimum quantity of dry DMF to complete the dissolution. 1/3 of the 1.8 ml (12.5 mmol) DIEA solution is added to this transparent solution in 1.8 ml of DCM. This is thoroughly homogenized and added to the dry resin. It is subjected to vigorous magnetic agitation for five minutes and the rest of the DIEA is added to the reaction; the mixture is allowed to react for forty minutes more. Then, 4 ml of dry MeOH are added and allowed to react for 10 minutes, after which the resin is filtered and the washings described below are carried out.
brand name
Sandostatin (Novartis).
Therapeutic Function
Antiulcer, Growth hormone inhibitor
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Clinical Use
Relief of symptoms of gastro-enteropancreatic endocrine tumours and acromegaly
Safety Profile
A poison by subcutaneous andintravenous routes. Human systemic effects: changes instructure or function of endocrine pancreas, liver functiontests impaired. When heated to decomposition it emitstoxic vapors of NOx and SOx
Drug interactions
Potentially hazardous interactions with other drugs
Ciclosporin: ciclosporin concentration reduced.
Metabolism
Extensive hepatic metabolism.1
Octreotide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 | xinshengkeji@xsmaterial.com | China | 1085 | 58 |
| Zhejiang Hangyu API Co., Ltd | +8617531972939 | anna@api-made.com | China | 2943 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 | Ethan@dornechem.com | China | 3096 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 | admin@oudaxin.com | China | 114 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| Shandong Huizhihan Supply Chain Co., Ltd | +8613363081709 | 3957328362@qq.com | China | 257 | 58 |
| Alpha Biopharmaceuticals Co., Ltd | +86-15542445688 | sales@alabiochem.com | China | 888 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Rixing Chemical CO.,LTD | +86-13237129059 -13237129059 | sales@rixingchemi.com | CHINA | 229 | 55 |
Related articles
- The uses and biological function of Octreotide
- Octreotide is a synthetic somatostatin analog with several palliative care indications, including managing ascites, bowel obst....
- Nov 28,2023
View Lastest Price from Octreotide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-28 | Octreotide
79517-01-4
|
US $50.00-43.00 / box | 1box | 99% | 10 Ton | Shandong Huizhihan Supply Chain Co., Ltd | |
 |
2024-11-28 | Octreotide
79517-01-4
|
US $1.00 / g | 1g | 99% | 100kg | Dorne Chemical Technology co. LTD | |
 |
2024-11-19 | Octreotide Acetate
79517-01-4
|
US $30.00-70.00 / mg | 99.87% | 10g | TargetMol Chemicals Inc. |
-

- Octreotide
79517-01-4
- US $50.00-43.00 / box
- 99%
- Shandong Huizhihan Supply Chain Co., Ltd
-

- Octreotide
79517-01-4
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD
-

- Octreotide Acetate
79517-01-4
- US $30.00-70.00 / mg
- 99.87%
- TargetMol Chemicals Inc.