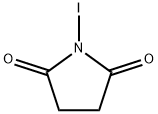
N-Iodosuccinimide
- CAS No.
- 516-12-1
- Chemical Name:
- N-Iodosuccinimide
- Synonyms
- NIS;1-iodopyrrolidine-2,5-dione;Iodosuccinimide;N-IodosucciniMide (NIS);1-IODO-2,5-PYRROLIDINEDIONE;N-iododiimide;succiniodimide;N-IODOSUCCINIMIDE;n-iodo-succinimid;N-lodosuccinimide
- CBNumber:
- CB0342078
- Molecular Formula:
- C4H4INO2
- Molecular Weight:
- 224.98
- MDL Number:
- MFCD00005512
- MOL File:
- 516-12-1.mol
- MSDS File:
- SDS
| Melting point | 202-206 °C(lit.) |
|---|---|
| Boiling point | 249.6±23.0 °C(Predicted) |
| Density | 2,245 g/cm3 |
| storage temp. | 2-8°C |
| solubility | Soluble in dioxane, tetrahydrfuran and acetonitrile. Insoluble in ether and carbon tetrachloride. |
| form | Crystalline Powder |
| pka | -2.57±0.20(Predicted) |
| color | White-yellow to brown |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,5045 |
| BRN | 113917 |
| Stability | Moisture Sensitive |
| InChIKey | LQZMLBORDGWNPD-UHFFFAOYSA-N |
| CAS DataBase Reference | 516-12-1(CAS DataBase Reference) |
| FDA UNII | 3COS3X3N4P |
| UNSPSC Code | 12352101 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H317-H319-H341-H410 | |||||||||
| Precautionary statements | P201-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38 | |||||||||
| Safety Statements | 26-36-37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | WN2817000 | |||||||||
| F | 8-9 | |||||||||
| Hazard Note | Harmful/Keep Cold/Moisture Sensitive | |||||||||
| TSCA | No | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29251995 | |||||||||
| NFPA 704 |
|
N-Iodosuccinimide price More Price(54)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 220051 | N-Iodosuccinimide 95% | 516-12-1 | 5g | $58.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 220051 | N-Iodosuccinimide 95% | 516-12-1 | 25g | $126 | 2024-03-01 | Buy |
| TCI Chemical | I0074 | N-Iodosuccinimide >98.0%(T) | 516-12-1 | 5g | $47 | 2024-03-01 | Buy |
| TCI Chemical | I0074 | N-Iodosuccinimide >98.0%(T) | 516-12-1 | 25g | $134 | 2024-03-01 | Buy |
| Alfa Aesar | A14320 | N-Iodosuccinimide, 97% | 516-12-1 | 5g | $53.1 | 2024-03-01 | Buy |
N-Iodosuccinimide Chemical Properties,Uses,Production
Chemical Properties
white-yellow to brown crystalline powder
Uses
N-Iodosuccinimide is used in the preparation of vinyl sulfones from olefins and benzenesulfinic acid. It acts as a source for I+ and involved in Hunsdiecker reactions for the conversion of cinnamic acids, and propiolic acids to the corresponding alfa-halostyrenes and 1-halo-1-alkynes respectively. It is also used to hydrolyze thioglycosides to 1-hydroxyglycosides with triluoroacetic acid. It is involved in the preparation of iodobenzene from 1,6-diynes. Further, it acts as an iodinating agent in chemical synthesis.
Uses
N-Iodosuccinimide is a iodo substituted succinimide that is used as an iodinating agent in chemical synthesis.
Uses
Iodination of ketones and aldehydes.
Highly substituted iodobenzenes prepared via an efficient 2-step process from 1,6-diynes.
Used with TFA to chemoselectively hydrolyze thioglycosides to 1-hydroxyglycosides.
Synthesis of vinyl sulfones from olefins and benzenesulfinic acid.
Preparation
To a solution of 39.2g of succinimide in 1200ml of boiling water was added 51.0g of freshly precipitated silver oxide, the mixture was filtered and the silver salt was allowed to crystallize. Filtration and washing with cold water furnished 45.0g of the silver salt of succinimide suitable for the iodination step.
The finely powdered salt (49.5g) was added in portions with stirring to a solution of 50.8g of iodine in 300ml of acetone, the temperature being maintained at 5-10°C. After decolorization (30 min.), the silver iodide was filtered, the solvent was removed under reduced pressure at room temperature and the residue was washed with ether, yielding 43g of N-iodosuccinimide with mp 189-191°C. An analytical sample (85% recovery) was obtained by dissolving in the minimum quantity of hot dioxane and precipitating with carbon tetrachloride; colorless needles, mp 200-201°C.
Ref: JACS 75, 3493 (1953)
Definition
ChEBI: N-iodosuccinimide is a five-membered cyclic dicarboximide compound having an iodo substituent on the nitrogen atom. It is a dicarboximide and a pyrrolidinone. It derives from a succinimide.
Reactions
N-Iodosuccinimide (NIS) is an iodinating agent that is used for various electrophilic iodinations and as source for iodine in radical reactions.
N-Iodosuccinimide is the least reactive of the N-haloamides in aromatic substitution. N-Iodosuccinimide does not act as an iodinating agent in pure dimethyl sulfoxide or N-ethylacetamide. On the other hand, butyl disulfide and a number of other sulfur-containing compounds are effective catalysts at low concentration for the iodination of guanosine derivatives using N-iodosuccinimide. This catalytic effect is observed when dimethyl sulfoxide is used as the solvent,but not when N-ethylacetamide is used.
https://www.organic-chemistry.org/chemicals/oxidations/n-iodosuccinimide-nis.shtm
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 3493, 1953 DOI: 10.1021/ja01110a055
Organic Syntheses, Coll. Vol. 5, p. 663, 1973
Tetrahedron Letters, 25, p. 233, 1984 DOI: 10.1016/S0040-4039(00)99848-4
Purification Methods
Crystallise it from dioxane/CCl4. It iodinates arenes in triflic acid. [Olah et al J Org Chem 58 3194 1993, Beilstein 21/9 V 544.]
N-Iodosuccinimide Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhu Nuowei chemistry Co., Ltd. | +86-0553-2911116-802 +undefined17756524438 | sales1@nuowei-chem.com | China | 1638 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 |
| Jinan Dexinjia Bio&Tech Co., Ltd | +86-0531-82375818 +86-15064153060 | sales@jndxj.com | China | 1267 | 58 |
| Hebei Fengmu Trading Co., Ltd. | +8613393234347 | Lyla@fengmuchem.com | China | 2946 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8805 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12830 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18147 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| airuikechemical co., ltd. | +86-18353166132 +86-18353166132 | sales02@airuikechemical.com | China | 983 | 58 |
Related articles
- N-Iodosuccinimide: A Versatile Reagent in Synthetic Chemistry
- N-Iodosuccinimide is prepared by reacting of N-silversuccinimide with I2 in anhydrous dioxane, removal of AgI by fitration, an....
- Dec 11,2024
- What are the chemical reactions involved in N-Iodosuccinimide?
- N-Iodosuccinimide (NIS) is an electrophilic iodide.
- May 9,2024
- N-Iodosuccinimide: Synthesis and applications
- N-Iodosuccinimide (NIS) is an iodinating agent that is used for various electrophilic iodinations and as source for iodine in ....
- May 19,2023
View Lastest Price from N-Iodosuccinimide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-02-06 | N-Iodosuccinimide
516-12-1
|
US $6.00 / KG | 1KG | More than 99% | 2000KG/MONTH | Hebei Saisier Technology Co., LTD | |
 |
2025-02-06 | N-Iodosuccinimide
516-12-1
|
US $10.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2025-02-05 | N-Iodosuccinimide
516-12-1
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd |
-

- N-Iodosuccinimide
516-12-1
- US $6.00 / KG
- More than 99%
- Hebei Saisier Technology Co., LTD
-

- N-Iodosuccinimide
516-12-1
- US $10.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- N-Iodosuccinimide
516-12-1
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd
516-12-1(N-Iodosuccinimide)Related Search:
1of4