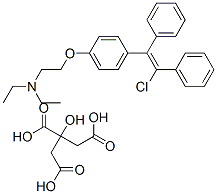
Clomiphene Citrate
- CAS No.
- 50-41-9
- Chemical Name:
- Clomiphene Citrate
- Synonyms
- CLOMID;CLOMIFENE CITRATE;clomifeno;CloMifene Citrate Capsules;SEROPHENE;mer41;mrl41;fertyl;omifin;dyneric
- CBNumber:
- CB0488051
- Molecular Formula:
- C32H36ClNO8
- Molecular Weight:
- 598.08
- MDL Number:
- MFCD00058322
- MOL File:
- 50-41-9.mol
- MSDS File:
- SDS
| Melting point | 116.5-118°C |
|---|---|
| storage temp. | 2-8°C |
| solubility | Slightly soluble in water, sparingly soluble in ethanol (96 per cent). |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Slightly soluble in water, methanol, chloroform (slightly), and DMSO (10 mM). Insoluble in ether. |
| BCS Class | 3/1 |
| InChIKey | PYTMYKVIJXPNBD-OQKDUQJOSA-N |
| SMILES | N(CC)(CC)CCOC1=CC=C(/C(/C2=CC=CC=C2)=C(\Cl)/C2=CC=CC=C2)C=C1.C(O)(=O)CC(CC(O)=O)(C(O)=O)O |
| CAS DataBase Reference | 50-41-9(CAS DataBase Reference) |
| FDA UNII | 1B8447E7YI |
| Proposition 65 List | Clomiphene Citrate |
| NCI Drug Dictionary | Clomid |
| IARC | 3 (Vol. 21, Sup 7) 1987 |
| EPA Substance Registry System | Clomiphene citrate (50-41-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H360FD |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| Hazard Codes | T |
| Risk Statements | 60-63 |
| Safety Statements | 53-36/37-45 |
| WGK Germany | 3 |
| RTECS | YE0875000 |
| HazardClass | IRRITANT |
| HS Code | 2922190900 |
| Toxicity | LD50 oral in rat: 5750mg/kg |
Clomiphene Citrate price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C6272 | Clomiphene citrate salt analytical standard | 50-41-9 | 1g | $89.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1140000 | Clomiphene citrate United States Pharmacopeia (USP) Reference Standard | 50-41-9 | 500mg | $436 | 2024-03-01 | Buy |
| Alfa Aesar | J63663 | Clomiphene citrate | 50-41-9 | 2g | $72.65 | 2024-03-01 | Buy |
| Alfa Aesar | J63663 | Clomiphene citrate | 50-41-9 | 10g | $149.65 | 2024-03-01 | Buy |
| Cayman Chemical | 16087 | Clomiphene (citrate) ≥98% | 50-41-9 | 1g | $32 | 2024-03-01 | Buy |
Clomiphene Citrate Chemical Properties,Uses,Production
Historical issue
Clomiphene citrate has been stated that 40 to 50 percent of successfully treated women conceive[2].
Unfortunately, results in men have been ambiguous and somewhat less favorable. The reasons for discrepancy between studies, which range from the mid 1960s to the present, are many. Various dosages (1 to 400 mg. daily) and treatment intervals (3 to 24 months) have been used. Selection of candidates provides another source of inconsistency. Criteria for inclusion into trial and pre-trial evaluation have varied markedly. Wives were not always evaluated for infertility" Furthermore here have been no well-defined standards for what constitutes significant improvement in sperm quality. In addition to an already capricious situation, there have been only a few placebo-controlled trials to date[7, 8].
Indication and dosage
Clomiphene citrate is used mainly in female infertility due to anovulation (e.g. due to polycystic ovary syndrome) to induce ovulation. Clomiphene citrate(CC )has also been employed for ovarian stimulation in ovulating women, mainly for idiopathic (unexplained) infertility and often combined with IUI. The rationale is presumably that Clomiphene citrate overcomes a subtle defect in ovulatory function or increases the number of mature follicles so increasing the likelihood of pregnancy[9]. Here the success rate has been, understandably, notably less than in anovulatory women.
CC is given orally in a dose of 50–250 mg per day for 5 days from day 2, 3, 4 or 5 of spontaneous or induced bleeding, starting with the lowest dose and increasing the dose in increments of 50 mg/day per cycle until an ovulatory cycle is achieved. The starting day of treatment, whether on day 2 or through day 5 of the cycle, does not influence the results[10]. Although 50 mg/day is the recommended dose in the first cycle, a meta-analysis of 13 published reports[11] suggests that only 46% will respond to this dose with ovulation, a further 21% will respond to 100 mg and another 8% will ovulate with 150 mg/day. There is no apparent advantage of using a daily dose of 150 mg that seems to significantly increase neither the ovulation rate nor follicular recruitment[12]. Some practitioners often use a starting dose of 100 mg/day from day 4 or 5, only resorting to 50 mg/day in the case of exquisite sensitivity or persistent cyst formation. The advantage of starting with a 100 mg daily dose rather than 50 mg is that it will cut down the number of ‘superfluous’ cycles of treatment until ovulation is achieved and until those resistant to CC are identified. It is difficult to state what effect, if any, this course of action has on the multiple pregnancy rates.
Mode of action
Clomiphene contains an unequal mixture of two isomers as their citrate salts, enclomiphene and zuclomiphene. Zuclomiphene is much the more potent of the two for induction of ovulation, accounts for 38% of the total drug content of one tablet and has a much longer half-life than enclomiphene, being detectable in plasma 1 month following its administration. Rostami-Hodjegan et al[11] have suggested that wide variability in the metabolism of the zuclomiphene component contributes to variability in response to the drug.
Clomiphene citrate is capable of inducing a discharge of FSH from the anterior pituitary and this is often enough to reset the cycle of events leading to ovulation into motion. The release of even small amounts of FSH into the system will often induce ovulation and pregnancy in a proportion of eu-estrogenic anovulatory women. This is achieved indirectly, through the action of Clomiphene citrate, a non-steroidal compound closely resembling an estrogen, in blocking hypothalamic estrogen receptors, signalling a lack of circulating estrogen to the hypothalamus and inducing a change in the pattern of pulsatile release of GnRH. Clomifene has no apparent progestational, androgenic, or antrandrogenic effects and does not appear to interfere with pituitary-adrenal or pituitary-thyroid function.
Adjuvant
In order to improve the outcome of treatment with CC, several adjuvants to CC treatment have been suggested. A correctly timed ovulation-triggering dose of HCG (5000– 10000 IU) is only theoretically warranted when the reason for a non-ovulatory response is that the LH surge is delayed or absent despite the presence of a well developed follicle. Although the routine addition of HCG at mid-cycle seems to add little to the improvement of conception rates[13], we have found it very useful, if given when an ultrasonically demonstrated leading follicle attains a diameter of 19–24 mm, for the timing of intercourse or IUI. The addition of dexamethazone as an adjunct to CC therapy in a dose of 0.5 mg at bedtime is said to suppress adrenal androgen secretion and induce responsiveness to CC in previous non-responders, mostly hyperandrogenic women with PCOS and elevated concentrations of dehydroepiandrosterone sulphate (DHEAS)[14]. However, glucocorticoid steroid therapy often induces side effects including increased appetite and weight gain, and should probably be reserved for women who have congenital adrenal hyperplasia as a cause for their anovulation.
Pharmacokinetics
Based on early studies with 14 C-labeled clomifene, the drug was shown to be readily absorbed orally in humans. The drug was shown to be readily absorbed orally in humans and excreted principally in the feces. Mean urinary excretion was approximately 8% with fecal excretion of about 42%.
Adverse reactions
Unpleasant side effects of CC are few and far between, although some women will complain of hot flushes and some of nausea. CC is, however, usually very well tolerated. While mild ovarian enlargement is relatively common, in almost 40 years of practice, it has never seen a full-blown ovarian hyperstimulation syndrome (OHSS) as a result of CC treatment. Occasional cyst formation can be treated conservatively.
Some specific side effects (rare) include bloating, stomach or pelvic pain, blurred vision, sensitivity of eyes to light, nausea or vomiting, nervousness, restlessness, tiredness, trouble in sleeping and mental depression[15, 16]. Some side effects of clomiphene may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them.
Precaution
You should not take this drug if you have the following conditions: abnormal vaginal bleeding; an ovarian cyst that is not related to polycystic ovary syndrome; past or present liver disease; a tumor of your pituitary gland; an untreated or uncontrolled problem with your thyroid or adrenal gland; or if you are pregnant.
You should not take it if you are already pregnant. Talk to your doctor if you have concerns about the possible effects of this medicine on a new pregnancy. Clomiphene can pass into breast milk and may harm a nursing baby. This medication may slow breast milk production in some women. Tell your doctor if you are breast-feeding a baby. Using clomiphene for longer than 3 treatment cycles may increase your risk of developing an ovarian tumor. Ask your doctor about your specific risk. Fertility treatment may increase your chance of having multiple births (twins, triplets). These are high-risk pregnancies both for the mother and the babies. Talk to your doctor if you have concerns about this risk.
References
- Goodman, L. S., Gilman, A. G. and Gilman, A. Z.: The Pharmacological Basis of Therapeutics, 6th ed. New York: MacMillan Publishing Co., Inc., p. 1432, 1980.
- Goodman L. S., Gilman, A. G. and Gilman, A. Z.: The Pharmacological Basis of Therapeutics, 6th ed. New York: MacMillan Publishing Co., Inc., p. 1433, 1980
- Attia GR, Rainey WE and Carr BR (2001) Metformin directly inhibits androgen production in human thecal cells. Fertil Steril 76,517–524.
- Mansfield R, Galea R, Brincat M, Hole D and Mason H (2003) Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril 79, 956–962.
- Mitwally MF and Casper RF (2001) Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril 75,305–309.
- Lopez E, Gunby J, Daya S, Parrilla JJ, Abad L and Balasch J (2004) Ovulation induction in women with polycystic ovary syndrome: randomized trial of clomiphene citrate versus low-dose recombinant FSH as first line therapy. Reprod Biomed Online 9,382–390.
- Foss, G. L., Tindall, V. R. and Birkett, J. P.: The treatment of subfertile men with clomiphene citrate. J. Reprod. Fertil., 32: 167, 1973.
- Ronnberg, L.: The effect of clomiphene citrate on different sperm parameters and serum hormone levels in preselected infertile men: a controlled double-blind cross-over study. Int. J. Androl., 3: 479, 1980.
- Guzick DS, Sullivan MW, Adamson GD, Cedars MI, Falk RJ, Peterson EP and Steinkampf MP (1998) Efficacy of treatment for unexplained infertility. Fertil Steril 70,207–213.
- Wu CH and Winkel CA (1989) The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril 52,564–568.
- Rostami-Hodjegan A, Lennard MS, Tucker GT and Ledger WL (2004) Monitoring plasma concentrations to individualize treatment with clomiphene citrate. Fertil Steril 81,1187–1193
- Dickey RP, Taylor SN, Curole DN, Rye PH, Lu PY and Pyrzak R (1997) Relationship of clomiphene dose and patient weight to successful treatment. Hum Reprod 12,449–453
- Agarwal SK and Buyalos RP (1995) Corpus luteum function and pregnancy rates with clomiphene citrate therapy: comparison of human chorionic gonadotrophin-induced versus spontaneous ovulation. Hum Reprod 10, 328–331.
- Daly DC, Walters CA, Soto-Albors, Tohan N and Riddick DH (1984) A randomized study of dexamethasone in ovulation induction with clomiphene citrate. Fertil Steril 41,844–848.
- Mauss, J., Mohnfeld, G. and Biirsch, G.: Synthetic LH-releasing factor and clomiphene stimulation in oligospermic males with normal FSH excretion. J. Reprod. Fertil., 40: 171, 1974.
- Espinasse, J.: Utilisation du citrate de clomiphene isolement ou en association avec !es androgenes dans le traitement des hypofertilities masculines idiopathiques. A propos de 7 4 cas. PhD dissertation, University of Toulouse.
Chemical Properties
White crystalline powder with a melting point range of 116.5-118°C. It has slight solubility in ethanol, chloroform, and water, but is insoluble in ether.
Originator
Clomid,Lepetit,Italy,1966
Uses
Clomiphene is a selective estrogen receptor modulator that impairs the activation of estrogen receptors (ERs) by 17β-estradiol. It potently binds both ERα and ERβ (Ki = 0.9 and 1.2 nM, respectively). Clomiphene enhances the release of gonadotropin-releasing hormone, stimulating the release of follicle-stimulating hormone and luteinizing hormone, culminating in ovulation.
Uses
Clomiphene Citrate is an unducer of ovulation. It is a selective estrogen receptor modulator, which acts by inhibiting the action of estrogen on the pituitary. Clomiphene Citrate is also an activator of PKC and Gonad-stimulating principle.
Manufacturing Process
A mixture of 20 g of 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylethanol
in 200 cc of ethanol containing an excess of hydrogen chloride was refluxed 3
hours. The solvent and excess hydrogen chloride were removed under
vacuum, and the residue was dissolved in a mixture of ethyl acetate and
methylene chloride. 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylethylene
hydrochloride was obtained, melting at 148° to 157°C. This hydrochloride salt
was treated with N-chlorosuccinimide in dry chloroform under reflux. The
product then obtained was converted to the free base and treated with citric
acid. The dihydrogen citrate salt of 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-
diphenylchloroethylene was obtained, melting at 116.5° to 118°C.
The intermediate 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylethanol was
obtained by treating 4-(β-diethylaminoethoxy)benzophenone with
benzylmagnesium chloride. It melted at 95° to 96°C.
brand name
Clomid (Sanofi Aventis); Serophene (Serono).
Therapeutic Function
Antiestrogen
Clomiphene Citrate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9314 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | ada@sh-teruiop.com | China | 875 | 58 | |
| Shanghai Getian Industrial Co., LTD | +86-15373193816 +86-15373193816 | mike@ge-tian.com | China | 269 | 58 |
| Rixing Chemical CO.,LTD. | +86-852-57055271 +8613237129059 | sales@rixingbiz.com | China | 232 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 7514 | 58 |
| Wuhan Cell Pharmaceutical Co., Ltd | +86-13129979210 +86-13129979210 | sales@cellwh.com | China | 376 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 615 | 58 |
Related articles
- Clomiphene Citrate for the Treatment of Hypogonadism
- Clomiphene Citrate is an effective alternative treatment for hypogonadism in men, preserving fertility and improving symptoms ....
- Jan 25,2024
- Clomiphene citrate: applications in the treatment of infertility
- Clomiphene citrate is an orally administered, non-steroidal anti-estrogen with ovulation-stimulating properties.The drug was a....
- Sep 20,2023
- What is Clomiphene Citrate?
- Clomiphene citrate (Clomiphene citrate, CC) is the first-line drug for ovulation stimulation in patients with PCOS so far. Bec....
- Sep 18,2021
View Lastest Price from Clomiphene Citrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-27 | Clomiphene Citrate
50-41-9
|
US $0.00 / Kg/Bag | 1KG | 98.0%~102.0%; USP36 | 1500kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Clomiphene citrate
50-41-9
|
US $45.00 / mg | 98.67% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Clomiphene citrate
50-41-9
|
US $45.00 / mg | 98.67% | 10g | TargetMol Chemicals Inc. |
-

- Clomiphene Citrate
50-41-9
- US $0.00 / Kg/Bag
- 98.0%~102.0%; USP36
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Clomiphene citrate
50-41-9
- US $45.00 / mg
- 98.67%
- TargetMol Chemicals Inc.
-

- Clomiphene citrate
50-41-9
- US $45.00 / mg
- 98.67%
- TargetMol Chemicals Inc.