Atazanavir
- CAS No.
- 198904-31-3
- Chemical Name:
- Atazanavir
- Synonyms
- Atv;Atazanavir iMpurity;CS-534;CS-2210;atazanvir;EOS-60363;Atazanavir;Aids057755;Aids-057755;Latazanavir
- CBNumber:
- CB0500863
- Molecular Formula:
- C38H52N6O7
- Molecular Weight:
- 704.86
- MDL Number:
- MFCD08435966
- MOL File:
- 198904-31-3.mol
| Melting point | 207-2090C |
|---|---|
| alpha | D -47° (c = 1 in ethanol) |
| Density | 1.178±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Ethanol (Slightly), Methanol (Slightly) |
| pka | 11.11±0.46(Predicted) |
| form | powder |
| color | white to beige |
| CAS DataBase Reference | 198904-31-3 |
| FDA UNII | QZU4H47A3S |
| ATC code | J05AE08 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P305+P351+P338 | |||||||||
| NFPA 704 |
|
Atazanavir price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1796 | Atazanavir ≥98% (HPLC) | 198904-31-3 | 5MG | $120 | 2023-06-20 | Buy |
| Sigma-Aldrich | SML1796 | Atazanavir ≥98% (HPLC) | 198904-31-3 | 25MG | $489 | 2023-06-20 | Buy |
| Cayman Chemical | 11733 | Atazanavir ≥95% | 198904-31-3 | 1mg | $39 | 2024-03-01 | Buy |
| Cayman Chemical | 11733 | Atazanavir ≥95% | 198904-31-3 | 5mg | $117 | 2024-03-01 | Buy |
| TRC | A790051 | Atazanavir | 198904-31-3 | 1mg | $55 | 2021-12-16 | Buy |
Atazanavir Chemical Properties,Uses,Production
Description
Atazanavir is an inhibitor of human immunodeficiency virus type 1 (HIV-1) protease, an enzyme that is essential for the processing of Gag and Gag-Pol polyproteins into structural and enzymatic proteins required for viral replication. It has a similar pharmacophore motif to the other six widely marketed HIV protease inhibitors, most of which are based upon a hydroxyethylamine template. Uniquely, it possesses an aza-peptide motif but maintains many similar pharmacophore elements including lipophilic moieties that presumably bind to S2, S1, S′1 , and S′2 positions. Atazanavir is pseudo-symmetric about the central template, incorporating D-tert-Leucine at both termini. This compound is synthesized in about seven steps, with a key coupling of the chiral epoxide (derived from phenylalanine and imparting one chiral center) and N-tert-boc-N′-(4-[2-pyridyl]benzyl)hydrazine. Removal of both tert-Boc groups and double acylation with methoxycarbonyl-tert-Leucine provides the product. Another synthesis of atazanavir entails ten steps and utilizes α-(tert-bocamino) phenylpropanal as a chiral intermediate. It is a potent inhibitor of indinavir-resistant and saquinavir-resistant strains of HIV-1 (IC50=0.03–0.1 and 0.04–0.1 μM, respectively). In 300 patients who had failed previous treatment, atazanavir (400 mg once daily) was compared to lopinavir (400 mg twice daily) and ritonavir (100 mg); both arms additionally receiving two non-reverse transcriptase inhibitors. After 24 weeks, HIV RNA levels of <400 copies/mL were noted in 61% of patients receiving atazanavir and 81% of those taking lopinavir/ritonavir. After 96 weeks of therapy with atazanavir, HIV RNA copy levels were found to be <400 and <50 in 80 and 58% of patients, respectively. A study of the cross-resistance profile relative to other protease inhibitors using a panel of 551 clinical isolates (without prior atazanavir exposure but with cross-resistance to one or two other protease inhibitors; the majority had resistance to nelfinavir) showed that greater than 80% retained susceptibility to atazanavir. All of the resistant isolates from patients taking atazanavir had an I50 L substitution. The recommended dosage of atazanavir is 400 mg once daily. It has a mean half-life range of 7.9–6.5 h with about 60% bioavailability and moderate plasma protein binding (86% albumin and 89% alpha-1- acid glycoprotein (AAG)). Atazanavir was well tolerated in clinical studies and it displayed minimal lipid modulation when tested in combination with two non-reverse transcriptase inhibitors. Atazanavir had no effect on total cholesterol, low-density lipoprotein, and triglyceride levels when compared with other protease inhibitors that caused sustained elevations in these lipid levels.
Chemical Properties
Crystalline Solid
Originator
Novartis (US)
Uses
enzyme inhibitor
Uses
Atazanavir is an inhibitor of HIV-1 protease (EC50 = 2.6 nM). In isolated cells, it has additive to moderately synergistic antiviral effects when combined with other antiretroviral drugs. As a result, it is commonly used in vivo in combination therapy for HIV-1 infection. Atazanavir competitively inhibits UDP-gluronosyltransferase, which conjugates bilirubin for clearance, leading to hyperbilirubinemia in a significant portion of those receiving atazanavir therapy.
Uses
Atazanavir is a novel azapeptide protease inhibitor (PI)
Uses
Atazanavir is a novel azapeptide HIV protease inhibitor (PI). Antiviral.
Definition
ChEBI: A heavily substituted carbohydrazide that is an antiretroviral drug of the protease inhibitor (PI) class used to treat infection of human immunodeficiency virus (HIV).
brand name
Reyataz (Bristol-Myers Squibb).
Acquired resistance
Mutations at positions 50 (I50L), 84 (I84V) and 88 (N88S) of the protease gene are associated with resistance.
General Description
Atazanavir is an antiretroviral agent that has been approvedby the FDA for use in combination with other anti-RTagents for the treatment of HIV infections. The drug is alwaysused in combination with RT inhibitors.
Pharmaceutical Applications
An azapeptide formulated as the sulfate for oral use.
Biochem/physiol Actions
Atazanavir is an antiviral HIV protease inhibitor.
Mechanism of action
Atazanavir is dosed orally once daily, thus reducing "pill burden," and it appears to have minimal impact on lipid parameters but does increase total bilirubin. The drug is well absorbed when administered orally with food (bioavailability, ~68%). The drug is highly bound to plasma protein (86%) and is metabolized by CYP3A isoenzyme. Atazanavir is a moderate inhibitor of CYP3A, and potential drug–drug interactions are possible with CYP3A inhibitors and inducers.
Pharmacokinetics
Oral absorption: c. 68%
Cmax 400 mg once daily: c. 3.15 μg/L
300 mg + ritonavir 100 mg once daily: c. 4.47 μg/L
Cmin 400 mg once daily: c. 0.27 μg/L
300 mg + ritonavir 100 mg once daily: c. 0.65 μg/L
Plasma half-life: c. 8.6 h (300 mg+ ritonavir
100 mg)
Volume of distribution: c. Not known/available
Plasma protein binding: c. 86%
Absorption
Administration with food enhances bioavailability and reduces pharmacokinetic variability. Absorption is dependent on gastric pH. It should be given separately from proton-pump inhibitors or H2-receptor antagonists. Buffered or entericcoated formulations should be given (with food) 2 h before or 1 h after co-administration of didanosine.
Distribution
It penetrates moderately well into the CNS. The semen:plasma ratio is 0.11–4.42. It is distributed into breast milk.
Metabolism
It is extensively metabolized by CYP3A4. Administration with ritonavir prevents metabolization and enhances the pharmacokinetic profile.
Excretion
Following a single 400 mg dose, 79% and 13% of the dose was recovered in the feces and urine, respectively. It should be used with caution in the presence of mild hepatic impairment and should not be used in patients with more severe hepatic impairment.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
Side effects
The most common adverse reactions (≥2%) are nausea, jaundice/ scleral icterus, rash, headache, abdominal pain, vomiting, insomnia, peripheral neurological symptoms, dizziness, myalgia, diarrhea, depression and fever.
target
HIV
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possibly increased levels of
amiodarone and lidocaine.
Antibacterials: concentration of both drugs
increased when given with clarithromycin; rifabutin
concentration increased - reduce dose of rifabutin;
rifampicin reduces atazanavir concentration - avoid;
avoid with telithromycin in severe renal and hepatic
impairment.
Anticoagulants: avoid with apixaban and
rivaroxaban.
Antidepressants: concentration reduced by St John’s
wort - avoid.
Antifungals: concentration increased by
posaconazole; concentration of voriconazole
increased or decreased, concentration of atazanavir
also reduced.
Antimalarials: avoid with artemether/lumefantrine;
may increase quinine concentration.
Antipsychotics: possibly inhibits metabolism of
aripiprazole - reduce dose of aripiprazole; possibly
increased concentration of pimozide and quetiapine
- avoid.
Antivirals: concentration reduced by boceprevir;
concentration of daclatasvir increased, reduce dose
of daclatasvir; absorption reduced by didanosine
tablets; concentration reduced by efavirenz -
avoid; concentration of elvitegravir increased
when atazanavir boosted with ritonavir - reduce
elvitegravir dose; concentration possibly reduced by
nevirapine - avoid; concentration of paritaprevir
increased; increased risk of ventricular arrhythmias
with saquinavir - avoid; concentration reduced
by tenofovir and tenofovir concentration possibly
increased; avoid with indinavir; concentration
of maraviroc increased, consider reducing
dose of maraviroc; possibly reduces telaprevir
concentration, also concentration of atazanavir
increased; concentration of tipranavir increased,
also concentration of atazanavir reduced; avoid
with elbasvir/grazoprevir, increased grazoprevir
concentration.
Anxiolytics and hypnotics: possibly increases
concentration of midazolam - avoid with oral
midazolam.
Calcium-channel blockers: concentration of
diltiazem increased - reduce dose of diltiazem;
possibly increased verapamil concentration.
Ciclosporin: possibly increased concentration of
ciclosporin.
Colchicine: possibly increases risk of colchicine
toxicity, avoid in hepatic or renal impairment.
Cytotoxics: possibly increases concentration of
axitinib, reduce dose of axitinib; possibly increases
concentration of bosutinib, avoid or reduce dose;
possibly increases concentration of crizotinib and
everolimus - avoid; avoid with cabazitaxel and
pazopanib; concentration of ibrutinib possibly
increased, reduce dose of ibrutinib; possibly inhibits
metabolism of irinotecan - increased risk of toxicity.
Dapoxetine: avoid concomitant use, increased risk of
toxicity.
Ergot alkaloids: possibly increased concentration of
ergot alkaloids - avoid.
Orlistat: absorption possibly reduced by orlistat.
Ranolazine: possibly increases ranolazine
concentration - avoid.
Sildenafil: possibly increased side effects of sildenafil.
Sirolimus: possibly increased concentration of
sirolimus.
Statins: avoid with simvastatin - increased risk of
myopathy; possibly increased risk of myopathy with atorvastatin, pravastatin and rosuvastatin - reduce
rosuvastatin dose.
Tacrolimus: possibly increased concentration of
tacrolimus.
Ticagrelor: possibly increases concentration of
ticagrelor - avoid.
Ulcer-healing drugs: concentration significantly
reduced by omeprazole and esomeprazole and
possibly other proton pump inhibitors - avoid;
concentration possibly reduced by histamine H2
antagonists.
Metabolism
Atazanavir is principally metabolised by CYP3A4
isozyme to oxygenated metabolites. Metabolites are
then excreted in the bile as either free or glucuronidated
metabolites. Additional minor metabolic pathways consist
of N-dealkylation and hydrolysis. Two minor metabolites
of atazanavir in plasma have been characterised. Neither
metabolite demonstrated in vitro antiviral activity.
Following a single 400 mg dose of [14C]-atazanavir, 79%
and 13% of the total radioactivity was recovered in the
faeces and urine, respectively. Unchanged drug accounted
for approximately 20% and 7% of the administered dose
in the faeces and urine, respectively.
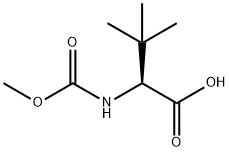


Atazanavir Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18153 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20286 | 58 |
| Alpha Biopharmaceuticals Co., Ltd | +86-15542445688 | sales@alabiochem.com | China | 888 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18775 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| Casorganics US Corp | +17326109938 | sales@casorganics.com | CHINA | 174 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
Related articles
- Uses and Side effects of Atazanavir
- Atazanavir (ATV) sulfate, (3S,8S,9S,12S)-3,12-bis(1,1-dimethylethyl)- 8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl....
- Apr 2,2022
View Lastest Price from Atazanavir manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-27 | Atazanavir
198904-31-3
|
US $0.00 / Kg/Bag | 1KG | 99%min | 100KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Atazanavir
198904-31-3
|
US $39.00-96.00 / mg | 99.95% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Atazanavir
198904-31-3
|
US $39.00-96.00 / mg | 99.95% | 10g | TargetMol Chemicals Inc. |
-

- Atazanavir
198904-31-3
- US $0.00 / Kg/Bag
- 99%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Atazanavir
198904-31-3
- US $39.00-96.00 / mg
- 99.95%
- TargetMol Chemicals Inc.
-

- Atazanavir
198904-31-3
- US $39.00-96.00 / mg
- 99.95%
- TargetMol Chemicals Inc.





