
Atazanavir synthesis
- Product Name:Atazanavir
- CAS Number:198904-31-3
- Molecular formula:C38H52N6O7
- Molecular Weight:704.86

Fan, Xing; Song, Yan-Li; Long, Ya-Qiu. An efficient and practical synthesis of the HIV protease inhibitor Atazanavir via a highly diastereoselective reduction approach. Organic Process Research & Development. State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Volume 12. Issue 1. Pages 69-75. 2008.
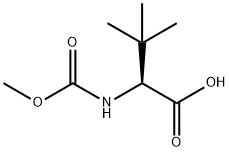
162537-11-3
410 suppliers
$6.00/250mg
![1-[4-(Pyridin-2-yl)phenyl]-4(S)-hydroxy-5(S)-2,5-diamino-6-phenyl-2-azahexane trihydrochloride](/CAS/20210305/GIF/437713-06-9.gif)
437713-06-9
3 suppliers
inquiry

198904-31-3
313 suppliers
$5.00/10mg
Yield:198904-31-3 74.2%
Reaction Conditions:
Stage #1:(S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid with thionyl chloride;triethylamine in dichloromethane at 42; for 3 h;
Stage #2:1-[4-(pyridine-2-yl)phenyl]-(5S)-2,5-diamino-(4S)-hydroxy-6-phenyl-2-azahexane in dichloromethane at 20;Solvent;Temperature;Concentration;
Steps:
2 Preparation of atazanavir monomer
To 100 mL of N-methoxycarbonyl-L-tert-leucine was placed in a 500 mL Erlenmeyer flask, 200 mL of methylene chloride was added and stirred to dissolve, and then triethylamine 70.5 g and 14.2 g (112. Ommol) of thionyl chloride were added and the temperature was raised to 42 ° C. The solvent was diluted with methylene chloride. The mixture was allowed to cool for 3 h. The reaction was completed and the temperature was reduced to room temperature. The triethylamine hydrochloride solid was removed by filtration.use.The solid product (32.58 g, 90 mmol) in Example 1 was dissolved in 50 mL of dichloromethane, and the filtrate was slowly added dropwise to the system at room temperature. The mixture was stirred overnight and the reaction was completed.The reaction solution was washed successively with 10% citric acid, 10% potassium carbonate, 10% sodium chloride and purified water to 300 mL of X, concentrated under reduced pressure to about dichloromethane content of less than about 20%, and 191.43 mL of methyl tert Butyl ether, beating, filtration, vacuum 50 ° C to dry 54.3 g of the crude azazavir monomer, and the crude product was purified in a 1: 1 by volume ratio of ethanol: water to give anazanavir monomer 47. 1g, yield 74.2%.
References:
Northeast Pharmaceutical Group Co., Ltd.;Bai Yuefei;Pi Changqiao;Han Xiaodan;Liu Dan;Sun Dongjun CN104356054, 2017, B Location in patent:Paragraph 0020-0022

857904-02-0
7 suppliers
inquiry
![Carbamic acid, N-[(1S)-2,2-dimethyl-1-[[[(1S)-1-(2R)-2-oxiranyl-2-phenylethyl]amino]carbonyl]propyl]-, methyl ester](/CAS/20210111/GIF/1192510-20-5.gif)
1192510-20-5
1 suppliers
inquiry

198904-31-3
313 suppliers
$5.00/10mg
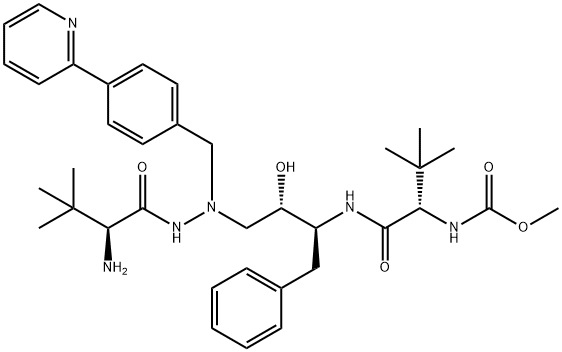
1028634-76-5
19 suppliers
inquiry
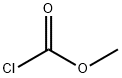
79-22-1
2 suppliers
$38.00/1000g

198904-31-3
313 suppliers
$5.00/10mg

127406-56-8
306 suppliers
$6.00/1g

198904-31-3
313 suppliers
$5.00/10mg
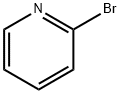
109-04-6
527 suppliers
$6.00/25g

198904-31-3
313 suppliers
$5.00/10mg






