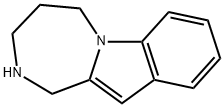
Azepindole
- CAS No.
- 26304-61-0
- Chemical Name:
- Azepindole
- Synonyms
- McN-2453;Azepindole;2,3,4,5-Tetrahydro-1H-[1,4]diazepino[1,2-a]indole;1H-[1,4]Diazepino[1,2-a]indole, 2,3,4,5-tetrahydro-
- CBNumber:
- CB11178073
- Molecular Formula:
- C12H14N2
- Molecular Weight:
- 186.25
- MDL Number:
- MOL File:
- 26304-61-0.mol
| Boiling point | 367.1±17.0 °C(Predicted) |
|---|---|
| Density | 1.19±0.1 g/cm3(Predicted) |
| pka | 10.85±0.20(Predicted) |
| FDA UNII | 6BB6FW9T8J |
Azepindole price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Biosynth Carbosynth | FA168210 | Azepindole | 26304-61-0 | 25mg | $300 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FA168210 | Azepindole | 26304-61-0 | 50mg | $400 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM1039886 | 1H,2H,3H,4H,5H-[1,4]DIAZEPINO[1,2-A]INDOLE 95.00% | 26304-61-0 | 5MG | $497.32 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FA168210 | Azepindole | 26304-61-0 | 100mg | $500 | 2021-12-16 | Buy |
| AK Scientific | 2623CA | Azepindole | 26304-61-0 | 50mg | $590 | 2021-12-16 | Buy |
Azepindole Chemical Properties,Uses,Production
Originator
Azependole,ZYF Pharm Chemical
Uses
Antidepressant.
Manufacturing Process
Ethyl indole-2-carboxylate (9.84 g, 0.052 mole) is dissolved in 150 ml
dioxane. Acrylonitrile (3.11 g, 0.0588 mole) and benzyltrimethylammonium
hydroxide (Triton B) (2 ml) are added and the mixture is warmed, with
stirring, at 50°-55°C for 45 min. The solution is cooled to room temperature
and stirred overnight. The reaction mixture is added to 500 ml water
containing 3 ml glacial acetic acid. The mixture is extracted with methylene
chloride, the organic layer washed with 2x25 ml water and dried over
magnesium sulfate. The solvent is removed under reduced pressure. The
remaining oil is dissolved in ether and filtered through alumina with ether as
eluant. Evaporation of the ether gives a solid. The product obtained is ethyl N-
(β-cyanoethyl)indole-2-carboxylate, melting point 84°-86°C.
A 2.42 g (0.01 mole) of ethyl N-(β-cyanoethyl)indole-2-carboxylate is
suspended in acetic anhydride (20 ml). The suspended compound is
hydrogenated on a Parr shaker in the presence of Raney nickel. The uptake of
hydrogen is complete after 1.5 h. The product recovered is recrystallized from
benzene-hexane. The product obtained is ethyl 1-(3-acetamidopropyl)indole-
2-carboxylate, melting point 83.5°-84.5°C.
Ethyl 1-(3-acetamidopropyl)indole-2-carboxylate (1.44 g, 0.005 mole) is
cyclized in the presence of sodium hydride (0.30 g, 0.00625 molar in hydride)
in xylene under reflux. A few drops of absolute ethanol are added after 1 h
reflux. Total reflux time is 2 h. The product recovered is crystallized from
benzenehexane. The product obtained is 2,3,4,5-tetrahydro-1H-1,4-
diazepino[1,2-a]indol-1-one, melting point 181°-183°C.
2,3,4,5-Tetrahydro-1H-1,4-diazepino[1,2-a]indol-1-one (6.0 g, 0.03 mole) in
200 ml of monoglyme is added dropwise to a stirred suspension of lithium
aluminum hydride (2.8 g, 0.08 mole) in 200 ml of monoglyme, and the
mixture heated under reflux overnight. Water is added to the cooled mixture,
and the mixture filtered. The filtrate is dried with anhydrous magnesium
sulfate and concentrated in vacuum, giving an oil which solidifies on standing
to give about 85% theoretical yield of the free amine, 2,3,4,5-tetrahydro-1H-
1,4-diazepino[1,2a]indol, melting point 75°-77°C.
Therapeutic Function
Antidepressant
Azepindole Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
Azepindole Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Carbosynth | -- | sales@carbosynth.com | United Kingdom | 6005 | 58 |
| Supplier | Advantage |
|---|---|
| Carbosynth | 58 |
26304-61-0(Azepindole)Related Search:
1of4