1,4,7-Triazacyclononane trihydrochloride
- CAS No.
- 58966-93-1
- Chemical Name:
- 1,4,7-Triazacyclononane trihydrochloride
- Synonyms
- 152505;TACN,3HCl;1,4,7-TriazacyclononanetriHCl;1,4,7-triazacyclononane, 3HCl;1,4,7-triazonane-1,4,7-triium;1,4,7-triazonane hydrochloride;1,4,7-Triazanonanehydrochloride;1,4,7-Triazacyclononane hydrochloride;1,4,7-TRIAZACYCLONONANE TRIHYDROCHLORIDE;Octahydro-1H-1,4,7-triazonine hydrochloride
- CBNumber:
- CB1153163
- Molecular Formula:
- C6H18Cl3N3
- Molecular Weight:
- 238.59
- MDL Number:
- MFCD00074887
- MOL File:
- 58966-93-1.mol
- MSDS File:
- SDS
| Melting point | 288 °C (dec.)(lit.) |
|---|---|
| storage temp. | Inert atmosphere,Room Temperature |
| form | Crystalline Powder |
| color | Off-white to yellow |
| BRN | 5287631 |
| InChIKey | HNPMVNQYFPWBKI-UHFFFAOYSA-N |
| CAS DataBase Reference | 58966-93-1 |
| UNSPSC Code | 12352005 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 3261 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 3-9 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
1,4,7-Triazacyclononane trihydrochloride price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 329495 | 1,4,7-Triazacyclononane trihydrochloride 97% | 58966-93-1 | 1g | $90 | 2024-03-01 | Buy |
| TCI Chemical | T1600 | 1,4,7-Triazacyclononane Trihydrochloride >98.0%(N)(T) | 58966-93-1 | 1g | $139 | 2024-03-01 | Buy |
| TCI Chemical | T1600 | 1,4,7-Triazacyclononane Trihydrochloride >98.0%(N)(T) | 58966-93-1 | 5g | $689 | 2024-03-01 | Buy |
| TRC | T767355 | 1,4,7-Triazacyclononane trihydrochloride | 58966-93-1 | 5g | $475 | 2021-12-16 | Buy |
| AK Scientific | X4707 | 1,4,7-Triazacyclononane trihydrochloride | 58966-93-1 | 5g | $746 | 2021-12-16 | Buy |
1,4,7-Triazacyclononane trihydrochloride Chemical Properties,Uses,Production
Chemical Properties
1,4,7-Triazacyclononane trihydrochloride is a off-white to yellow crystalline powder that It is a chelate ring with six phosphate groups and six nitrogen atoms. It has been shown to behave as an irreversible oxidation catalyst for carboxylate and amine molecules. This molecule also has redox potentials in the range of -0.35 to -0.5 volts and can reversibly oxidize inorganic acids with strong electron-donating properties such as phosphoric acid, nitric acid, and sulfuric acid.
Uses
1,4,7-Triazacyclononane trihydrochloride is a possible reagent for compleximetric titrations with a high cation-binding selectivity. It is also used in the organic synthesis of ligands and compounds requiring a triazacyclononane group.
Application
1,4,7-Triazacyclononane trihydrochloride can be used as a reagent to prepare:
Nonadentate ligand, 1,4,7-tris[(6-carboxypyridin-2-yl)methyl]-1,4,7-triazacyclononane (H3tpatcn), which is used to prepare lanthanide complexes with high water solubility and rigid C3 symmetric structures.
Dansyl cryptands as fluorescent indicators via amination of (bromobenzyl)triazacycloalkane and oxadiamines.
[1,4,7-tri(3-butenyl)-1,4,7-triazacyclononane and 1,4,7-tri(2-propenyl)-1,4,7-triazacyclononane] by reacting with corresponding alkenyl halide.
storage
This nitrogen crown ether analog, also known as octahydro-1h-1,4,7-triazonine trihydrochloride should be stored in a cool and dark place. Keep under inert gas and protect from moisture. 1,4,7-Triazacyclononane Trihydrochloride is known to be incompatible with oxidizing agents and should not be stored or handled in their vicinity.
Preparation
Synthesis of 1,4,7-Triazacyclononane trihydrochloride (tacn.3HCl)
A round bottom flask (1 L) was charged with 18M H2SO4 (450 mL) and 1,4,7-tris(p-toluenesulfonyl)-1,4,7-triazacyclononane (4) (138.8 g, 0.234 mol) added in small portions (approximately 10 g every 5 min). The mixture was heated with stirring in a heat block for 3 days at 120°C. The resulting black solution was cooled to room temperature and added dropwise using a dropping funnel to a vigorously stirred mixture of cold absolute EtOH/Et2O (1.5 L/900 mL) cooled in an ice bath. An overhead stirrer was used to ensure efficient stirring. A sticky hygroscopic brown/grey precipitate formed, which was isolated quickly by vacuum filtration and immediately dissolved in de–ionised H2O (1 L). The mixture was heated for 2 h at 60°C. The mixture was then cooled to room temperature, filtered through Celite and the resulting solution concentrated to 250 mL under reduced pressure at 65°C. Conc. HCl (200 mL) was added followed by absolute EtOH until the solution became cloudy. The mixture was stored at 4°C overnight to promote precipitation. The white precipitate was collected by filtration and washed with ice cold absolute EtOH (3 x 50 mL), followed by Et2O (2 x 50 mL) to yield tacn.3HCl (5) as a white crystalline powder. Yield 44.9 g (80%).
mp. 268.1–270.0°C (Lit.4 mp. 280–281°C). 1H NMR (300 MHz, D2O) δH 3.48 (12H, s, CH2). 13C NMR (75 MHz, D2O) δC 43.1 (CH2). ESI–MS m/z [M + H]+ 130.1. The 1H and 13C NMR spectral data were consistent with literature data.
Using iSUSTAIN(TM) to validate the chemical attributes of different approaches to the synthesis of tacn and bridged bis(tacn) ligands
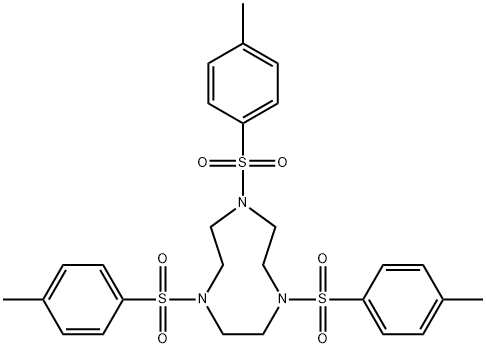
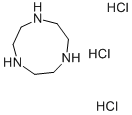
1,4,7-Triazacyclononane trihydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21631 | 55 |
| Fluoropharm Co., Ltd. | +86-0571-85586753; +8613336034509 | sales@fluoropharm.com | China | 1376 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29863 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | 1015@dideu.com | China | 3939 | 58 |
| Tangshan Moneide Trading Co., Ltd. | +86-315-8309571 +8615633399667 | sales@moneidechem.com | China | 574 | 58 |
View Lastest Price from 1,4,7-Triazacyclononane trihydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-24 | 1,4,7-Triazacyclononane trihydrochloride
58966-93-1
|
US $0.00-0.00 / kg | 1kg | 97% | 1000kg | Suzhou Laing Biotechnology Co.,Ltd | |
 |
2022-10-11 | 1,4,7-Triazacyclononane Trihydrochloride
58966-93-1
|
US $0.00-0.00 / kg | 1kg | 98% | 1Ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2019-07-06 | 1,4,7-TRIAZACYCLONONANE TRIHYDROCHLORIDE
58966-93-1
|
US $1.00 / kg | 1kg | 99% | 100KG | Career Henan Chemical Co |
-

- 1,4,7-Triazacyclononane trihydrochloride
58966-93-1
- US $0.00-0.00 / kg
- 97%
- Suzhou Laing Biotechnology Co.,Ltd
-

- 1,4,7-Triazacyclononane Trihydrochloride
58966-93-1
- US $0.00-0.00 / kg
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- 1,4,7-TRIAZACYCLONONANE TRIHYDROCHLORIDE
58966-93-1
- US $1.00 / kg
- 99%
- Career Henan Chemical Co





