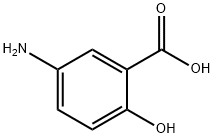
5-Aminosalicylic acid
- CAS No.
- 89-57-6
- Chemical Name:
- 5-Aminosalicylic acid
- Synonyms
- MESALAZINE;MESALAMINE;5-ASA;5-AMINO-2-HYDROXYBENZOIC ACID;Asacol;rowasa;3-Carboxy-4-hydroxyaniline;Canasa;Pentasa;Mesalmine
- CBNumber:
- CB1480481
- Molecular Formula:
- C7H7NO3
- Molecular Weight:
- 153.14
- MDL Number:
- MFCD00007877
- MOL File:
- 89-57-6.mol
- MSDS File:
- SDS
| Melting point | 275-280 °C (dec.) (lit.) |
|---|---|
| Boiling point | 276.03°C (rough estimate) |
| Density | 1.3585 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5500 (estimate) |
| Flash point | 279-281°C |
| storage temp. | 2-8°C |
| solubility | Soluble in dimethyl sulfoxide. |
| pka | 2.74, 5.84(at 25℃) |
| form | tablets |
| color | off-white to gray |
| PH | 4.0-4.1 (0.8g/l, H2O, 20℃) |
| PH Range | Non-B uorescence (3.1) to light green B uorescence (4.4) |
| Water Solubility | <0.1 g/100 mL at 21 ºC |
| Decomposition | 279-281 ºC |
| Merck | 14,5904 |
| BRN | 2090421 |
| BCS Class | 4 |
| Stability | Stable. Incompatible with acids, acid anhydrides, acid chlorides, chloroformates, strong oxidizing agents. |
| Major Application | Detergent, hair dyes, prevention of colorectal cancer, treating inflammatory bowel disease, autoimmune disorders, gastrointestinal inflammation, chemokine-mediated diseases, mucosal tissue disorder, sleep disorders, rectoanal tenesmus, ulcerative colitis |
| InChIKey | KBOPZPXVLCULAV-UHFFFAOYSA-N |
| LogP | 0.98 at 25℃ |
| CAS DataBase Reference | 89-57-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 4Q81I59GXC |
| NCI Drug Dictionary | Asacol |
| ATC code | A07EC02 |
| NIST Chemistry Reference | Mesalamine(89-57-6) |
| EPA Substance Registry System | Benzoic acid, 5-amino-2-hydroxy- (89-57-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P305+P351+P338-P261-P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313-P280a-P304+P340-P405-P501a | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38-52/53 | |||||||||
| Safety Statements | 26-36-24/25-61-37/39 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | VO1400000 | |||||||||
| F | 8-10-23 | |||||||||
| Autoignition Temperature | 280 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29225000 | |||||||||
| Toxicity | LD50 orally in Rabbit: 2800 mg/kg LD50 dermal Rat > 5000 mg/kg | |||||||||
| NFPA 704 |
|
5-Aminosalicylic acid price More Price(49)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | BP1075 | Mesalazine British Pharmacopoeia (BP) Reference Standard | 89-57-6 | 125MG | $257 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1392705 | Mesalamine United States Pharmacopeia (USP) Reference Standard | 89-57-6 | 200mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.20095 | 5-Amino-2-hydroxybenzoic acid for synthesis | 89-57-6 | 250g | $97.3 | 2022-05-15 | Buy |
| Sigma-Aldrich | 18858 | 5-Aminosalicylic acid analytical standard | 89-57-6 | 100mg | $55.2 | 2021-12-16 | Buy |
| TCI Chemical | A0317 | 5-Aminosalicylic Acid >98.0%(T) | 89-57-6 | 25g | $27 | 2024-03-01 | Buy |
5-Aminosalicylic acid Chemical Properties,Uses,Production
Indication
It is used for the treatment of active ulcerative proctitis.
References
- Martin F. Oral 5-aminosalicylic acid preparations in treatment of inflammatory bowel disease: an update. Dig Dis Sci 1987; 32 (12 Suppl.): 57S-63S
- Azad Khan AK, Piris J, Truelove SC. An experiment ot determine the active therapeutic moiety of sulphasalazine. Lancet 1979; II (8044): 892-5
- Schröder H, Price E, Evans DA. Acetylator phenotype and adverse events of sulphasalazine in healthy subjects. Gut 1972; 13 (4): 278-84
- Haagen Nielsen O, Bondesen S. Kinetics of 5-aminosalicylic acid after jejunal instillation in man. Br J Clin Pharmacol 1983; 16 (6): 738-40
- Ireland A, Jewell DP. Mechanism of action of 5-aminosalicylic acid and its derivatives. Clin Sci 1990; 78: 119-25
- Greenfield SM, Punchard NA, Teare JP, et al. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther 1993; 7: 369-83
- Travis SPL, Jewell DP. Salicylates for ulcerative colitis – their mode of action. Pharmacol Ther 1994; 63: 135-61
- Schmidt C, Fels T, Baumeister B, et al. The effect of 5aminosalicylate and para-aminosalicylate on the synthesis of prostaglandin E2 and leukotriene B4 in isolated colonic mucosal cells. Curr Med Res Opin 1996; 13 (7): 417-25
- Capasso F, Tavares IA, BennettA. Release of platelet-activating factor (PAF) from human colon mucosa and its inhibition by 5-aminosalicylic acid. Drugs Exp Clin Res 1991; 17: 351-3
- Rachmilewitz D, Karmeli F, Schwartz LW, et al. Effect of aminophenols (5-ASA and 4-ASA) on colonic interleukin-1 generation. Gut 1992; 33: 929-32
- Di Paolo MC, Merrett MN, Crotty B, et al. 5-Aminosalicylic acid inhibits the impaired epithelial barrier function induced by gamma interferon. Gut 1996; 38: 115-9
- Vainio H, Morgan G. Non-steroidal anti-inflammatory drugs and the chemoprevention of gastrointestinal cancers. Scand J Gastroenterol 1998; 33: 785–9.
- Bus PJ, Nagtegaal ID, Verspaget HW, et al. Mesalazine-induced apoptosis of colorectal cancer: on the verge of a new chemopreventive era? Aliment Pharmacol Ther 1999; 13: 1397–402.
- Reinacher-Schick A, Seidensticker F, Petrasch S, et al. Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy 2000; 32: 245–54.
- Egan LJ, Mays DC, Huntoon MP, et al. Inhibition of interleukin-1-stimulated NF-jB RelA ⁄ p65 phosphorylation by mesalazine is accompanied by decreased transcriptional activity. J Biol Chem 1999; 274: 26448–53.
- Bondesen S, Hegnhoj J, Larsen F, et al. Pharmacokinetics of 5-aminosalicylic acid in man following administration of intravenous bolus and Per Os slow-release formulation. Dig Dis Sci 1991; 36: 1735-40
- Daneshmend TK, Hendrickse M, Salzmann M, et al. Does systemic absorption of 5-aminosalicylic acid from olsalazine (Dipentum®) and mesalazine (Asacol® and Pentasa®) differ significantly in ulcerative colitis?[abstract]. Gut 1994; 35 Suppl. 4: 233
- Staerk-Laursen L, Stokholm M, Bukhave K, et al. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut 1990; 31: 1271-6
- Christensen LA, Rasmussen SN, Hansen SH. Disposition of 5-aminosalicylic acid and N-acetyl-5-aminosalicylic acid in fetal and maternal body fluids during treatment with different 5-aminosalicylic acid preparations. Acta Obstet Gynecol Scand 1994; 74: 399-402
- Lauritsen K, Laursen LS, Rask-Madsen J. Clinical pharmacokinetics of drugs used in the treatment of gastrointestinal diseases (Part II). Clin Pharmacokinet 1990; 19: 94-125
- Rasmussen SN, Bondesen S, Hvidberg EF, et al. 5-Aminosalicylic acid in a slow-release prepararation: bioavailability, plasma level, and excretion in humans. Gastroenterolo 1982; 83: 1062-70
- Christensen LA, Fallingborg J, Abildgaard K, et al. Topical and systemic availability of 5-amino-salicylate: comparisons of three controlled release preparations in man. Aliment Pharmacol Ther 1990; 4: 523-33
- Christensen LA, Fallingborg J, Jacobsen BA, et al. Comparative bioavailability of 5-aminosalicylic acid from a controlled release preparation and an azo-bond preparation. Aliment Pharmacol Ther 1994; 8: 289-94
- Hanauer S, Schwartz J, RobinsonM, et al.Mesalamine capsules for the treatment of active ulcerative colitis: results of a controlled trial. Am J Gastroenterol 1993; 88: 1188-97
- Miner P, Hanauer S, Robinson M, et al. Safety and efficacy of controlled-release mesalamine for maintenance of remission in ulcerative colitis. Dig Dis Sci 1995; 40: 296-304
- Singleton JW, Hanauer SB, Gitnick GL, et al. Mesalamine capsules for the treatment of active Crohn’s disease: results of a 16-week trial. Pentasa Crohn’s Disease Study Group[see comments]. Gastroenterology 1993; 104: 1293-301
- Hanauer SB, Krawitt EL, Robinson M, et al. Long-term management of Crohn’s disease with mesalamine capsules (Pentasa ®). Am J Gastroenterol 1993; 88: 1343-51
Description
5-Aminosalicylic acid (5-ASA), also known as mesalazine or mesalamine, is a metabolite and potential pharmacologically active component of sulphasalazine, a drug used in the treatment of Crohn’s disease and ulcerative colitis. However, the mechanism by which this drug works has not been established. In whole blood assays, 5-ASA proves to be a weak, non-selective inhibitor of both COX-1 and COX-2 with IC50 values of 410 and 61 μM, respectively. In ionophore-stimulated colonic mucosal cells, 1 mM 5-ASA does not inhibit prostaglandin E2 (PGE2) production, but does reduce leukotriene B4 (LTB4) synthesis approx. 50%. In ionophore-stimulated human leukocytes, 400 μM 5-ASA reduces LTB4 production approximately 20%. 5-ASA does not inhibit 15-hydroxy PGDH at concentrations up to 50 μM.
Chemical Properties
Off-White to pink crystals, melting point about 280 ℃ (decomposition). Soluble in hydrochloric acid, slightly soluble in hot water, and slightly bath in cold water or ethanol. In the manufacture of light-sensitive paper, azo and sulfur dyes.
Originator
Radcliffe Infirmary (United Kingdom)
Uses
5-Aminosalicylic acid is used in the preparation of gastrointestinal anti-inflammatory agents. It is a metabolite of sulfasalazine. It acts as a drug involved in the treatment of Crohn's disease and ulcerative colitis. Further, it is used to make dyes and light-sensitive papers.
Definition
ChEBI: Mesalamine is a monohydroxybenzoic acid that is salicylic acid substituted by an amino group at the 5-position. It has a role as a non-steroidal anti-inflammatory drug. It is an aromatic amine, an amino acid, a member of phenols, a monocarboxylic acid and a monohydroxybenzoic acid. It is functionally related to a salicylic acid. It is a conjugate acid of a mesalaminate(1-).
Preparation
Preparation by reduction of m-nitrobenzoic acid with Zn dust and HCl.
Application
5-Aminosalicylic acid (5-ASA) has been used to synthesize 5-formyl-aminosalicylate-inulin to quantify its release during in vitro digestion and fermentation and compare the in vitro fermentation properties of the conjugated inulin to native inulin. It is also used in cell adhesion assay to study its effects on E-cadherin glycosylation and membranous turnover. This compound is used to evaluate its effects on the neutrophilic inflammation index (NII) phenotype to study the effectiveness of the high cholesterol diet-gut inflammation (HCD-GI) platform. 5-Aminosalicylic acid is a peroxidase substrate suitable for use in ELISA procedures. This substrate produces a soluble end product that is brown in color and can be read spectrophotometrically at 450 nm. The reaction may be stopped with 3 N NaOH and read at 550 nm.
Manufacturing Process
Procedure A: To 5-nitrosalicylic acid potassium salt (55 g, 246 mmol) dissolved in water (200 mL) was added potassium hydroxide pellets to reach pH 11.5. To this solution 2 g of Raney nickel were added. The mixture was heated-up to reflux and hydrazine hydrate (40 mL, 80% in water, 64 mmol) was added dropwise during 3-4 hrs. The reflux was maintened until HPLC showed the disappearance of the starting material and the complete reduction of 5-nitrosalicylic acid (3-4 hrs). The hot mixture was filtered under nitrogen and the solution was collected. The solution was cooled to 40°C and the pH was adjusted to 2.3 by addition of 35% HCl aqueous solution. The precipitation of 5-aminosalicylic acid occurred. The solution was cooled at 0°C, and after standing at this temperature for 2 hr, the precipitate was filtered, washed with water, and dried at 60-70°C. 5-Aminosalicylic acid was obtained in 89% yield.
Procedure B: To 5-nitrosalicylic acid potassium salt (55 g, 246 mmol) dissolved in water (200 mL) was added potassium hydroxide pellets to reach pH 11.5. The solution was charged in a stainless steel autoclave and 2 g of Raney nickel are added. Hydrogen was introduced into the autoclave reaching a pressure of 8 atm. The mixture was heated-up to 100°C. The temperature was maintained until HPLC-test 5-aminosalicylic acid showed the disappearance of the starting material and the complete reduction of 5- aminosalicylic acid (6-8 hrs). Hydrogen was purged and replaced by nitrogen. The hot mixture was filtered under nitrogen, the filtrate was cooled to 40°C, and the pH was adjusted to 2.3 by addition of 35% HCl aqueous solution. The precipitation of the 5-aminosalicylic acid occurred. The solution was cooled at 0°C, and after standing at this temperature for 2 hr, the precipitate was filtered, washed with ion depleted water, and dried at 60-70°C.
brand name
SALOFALK
Therapeutic Function
Antibacterial
General Description
Odorless white to pinkish crystals or purplish-tan powder. Aqueous solutions acidic (pH approximately 4.1 at 0.8 mg/L water) .
Air & Water Reactions
Sensitive to moisture. Water insoluble.
Reactivity Profile
5-Aminosalicylic acid is incompatible with acids, acid chlorides, acid anhydrides, chloroformates and strong oxidizers.
Fire Hazard
Flash point data for 5-Aminosalicylic acid are not available; however, 5-Aminosalicylic acid is probably combustible.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
5-Aminosalicylic acid (5-ASA) is a first-line medicine, used to treat inflammatory bowel diseases like ulcerative colitis (UC). It has a high-efficiency rate in maintenance and induction of remission. 5-ASA is an active component of sulfasalazine and also consists of the carbohydrate polymer, inulin. It might exhibit anti-oxidant activity to lessen tissue injury. 5-ASA is vital for the prevention of T cell activation and proliferation. It negatively regulates cyclooxygenase and lipoxygenase pathways and lowers the formation of prostaglandins and leukotrienes. 5-ASA stimulates the membranous expression of E-cadherin and boosts intercellular adhesion.
Clinical Use
Induction and maintenance of remission in ulcerative colitis
Safety Profile
Poison by intraperitoneal route.Moderately toxic by ingestion. Human systemic effects byingestion: hypermotility, diarrhea, dermatitis, increasedbody temperature. When heated to decomposition it emitstoxic fumes of NOx.
Metabolism
The absorbed part of mesalazine is almost completely acetylated in the gut wall and in the liver to acetyl-5- aminosalicylic acid. The acetylated metabolite is excreted mainly in urine by tubular secretion, with traces of the parent compound.
Purification Methods
It crystallises as needles from H2O containing a little NaHSO3 to avoid aerial oxidation to the quinone-imine. The Me ester gives needles from *C6H6, m 96o, and the hydrazide has m 180-182o (from H2O). [Fallab et al. Helv Chim Acta 34 26 1951, Shavel J Amer Pharm Assoc 42 402 1953, Beilstein 14 IV 2058.]
5-Aminosalicylic acid Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Zhejiang Kinso Technology Co., LTD. | +8619857958369 | sales@kinsopharma.com | China | 54 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | niki@zlchemi.com | China | 724 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
Related articles
- The Pharmacokinetics and toxicity of Mesalazine
- Mesalazine is a medication commonly used for the treatment of inflammatory bowel disease. Over the years, there has been signi....
- Nov 7,2023
- A useful anti-inflammatory agent: Mesalazine
- Mesalazine is an anti-inflammatory agent, structurally related to salicylates, which is active in inflammatory bowel disease.
- Nov 7,2023
Related Qustion
- Q:What is the Mechanism of action of 5-Aminosalicylic acid
- A:In vitro, 5-ASA shares many pharmacological properties of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin.
- Aug 19,2024
View Lastest Price from 5-Aminosalicylic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | 5-Aminosalicylic acid (Mesalazine)
89-57-6
|
US $0.00-0.00 / Kg/Drum | 1KG | 98.5%~101.5%; USP40 | 15tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-22 | Mesalazine
89-57-6
|
US $0.00-0.00 / kg | 1kg | 99% | 20tons | Sinoway Industrial co., ltd. | |
 |
2024-11-21 | 5-Aminosalicylic Acid
89-57-6
|
US $79.00-38.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co Ltd |
-

- 5-Aminosalicylic acid (Mesalazine)
89-57-6
- US $0.00-0.00 / Kg/Drum
- 98.5%~101.5%; USP40
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Mesalazine
89-57-6
- US $0.00-0.00 / kg
- 99%
- Sinoway Industrial co., ltd.
-

- 5-Aminosalicylic Acid
89-57-6
- US $79.00-38.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co Ltd