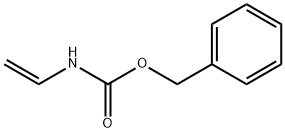
Benzyl vinylcarbamate
- CAS No.
- 84713-20-2
- Chemical Name:
- Benzyl vinylcarbamate
- Synonyms
- benzyl vinylcarbamate;N-Cbz-ethenamine;benzyl ethenylcarbamate;BENZYL-N-VINYLCARBAMATE;N-Vinyl-O-benzyl urethane;O-Benzyl-N-vinylcarbamate;benzyl N-ethenylcarbamate;Phenylmethyl ethenylcarbamate;Carbamic acid, N-ethenyl-, phenylmethyl ester
- CBNumber:
- CB1679005
- Molecular Formula:
- C10H11NO2
- Molecular Weight:
- 177.2
- MDL Number:
- MFCD04114067
- MOL File:
- 84713-20-2.mol
- MSDS File:
- SDS
| Melting point | 43-44°C |
|---|---|
| Boiling point | 272℃ |
| Density | 1.089 |
| Flash point | 118℃ |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Solid |
| pka | 11.04±0.46(Predicted) |
| color | White to Off-White |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36/37/39 |
Benzyl vinylcarbamate price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | B316510 | BenzylVinylcarbamate | 84713-20-2 | 2.5g | $305 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB141802 | Benzyl Vinylcarbamate | 84713-20-2 | 2g | $400 | 2021-12-16 | Buy |
| AK Scientific | 3024AC | Benzylvinylcarbamate | 84713-20-2 | 2g | $590 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB141802 | Benzyl Vinylcarbamate | 84713-20-2 | 1g | $250 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB141802 | Benzyl Vinylcarbamate | 84713-20-2 | 100mg | $80 | 2021-12-16 | Buy |
Benzyl vinylcarbamate Chemical Properties,Uses,Production
Uses
Benzyl Vinylcarbamate is used as a reagent in the synthesis of CF3- or -?CF2-?substituted tetrahydroquinolines.
Preparation
A 1-L flask was equipped with a variable speed pump, a mechanical stirrer, a temperature controller, a 4" (10 cm) column packed with ceramic saddles, a distillation head, a spiral condenser (cooled with water at 10–15 ℃), and a receiver. The flask was charged with toluene (150–200 mL) and phenothiazine (0.5 g) and the solution was heated to 105–110 ℃. The receiver was charged with benzyl alcohol (86 g, 0.8 mol), phenothiazine (0.05 g), and triethylamine (0.1–0.3 g). This mixture was cooled in ice and stirred. A solution of acryloyl azide (1 mol), prepared as described above, was pumped into the distillation flask over a period of 4–5 h, maintaining the pot temperature at 105–110 ℃ with a heating mantle. The vapor temperature varied, depending on the rate of addition of the azide, but was in the range 80–100 ℃. The distillate was passed directly into the benzyl alcohol mixture. After the addition of acryloyl azide, the distillation continued, generating a further 10–20 mL of toluene. The receiver was then removed from the distillation set-up, and its contents were stirred at 0–5 ℃ for 1– 2 h. The product mixture was then allowed to gradually warm to room temperature and was stirred until HPLC analysis indicated complete reaction. The mixture was then concentrated in vacuo to a weight of 200–250 g. The residue was treated with heptane (300–350 mL) and cooled to 15 ℃ with stirring. A few seed crystals of benzyl N-vinyl carbamate 810 were added, and the mixture was stirred for 2–3 h. The product was collected by filtration, washed with heptane, and dried in vacuo. Yield 115–128 g (65–72%); mp 41–44 ℃.
Application
Benzyl-N-vinyl carbamate (Z-vinylamine; Benzyl vinylcarbamate) is a valuable synthetic intermediate. This compound undergoes alkylation readily on the carbon R to the nitrogen, a property that has been used in the synthesis of a-lactam antibiotics. Z-vinylamine can be readily polymerized into polyvinyl amine derivatives[1].
Synthesis Reference(s)
The Journal of Organic Chemistry, 54, p. 4649, 1989 DOI: 10.1021/jo00280a036
References
[1] Govindan, Cheruthur K. “An Improved Process for the Preparation of Benzyl-N-vinyl Carbamate1.” Organic Process Research Development 6 1 (2001): 74–77.
Benzyl vinylcarbamate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Guangzhou Yuheng Pharmaceutical Technology Co., Ltd | +8613580539051 | joe@yuhengpharm.com | CHINA | 21142 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10319 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30239 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 | ada@ipurechemical.com | CHINA | 3461 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25356 | 58 |
| JUYE XIANDAI fine chemistry Co.,Ltd | +86-18958038633; +8618958038633 | tony@sdhypharma.com | China | 64 | 58 |
| Aromsyn Co., Ltd. | +86-0571-85585865 +8613336024896 | CB@aromsyn.com | China | 19183 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8467 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
Related articles
- How to synthesize Benzyl vinylcarbamate?
- Govindan developed an improved method that can be easily scaled up and has been designed to prepare benzyl-N-vinyl carbamate.
- Mar 26,2024
84713-20-2(Benzyl vinylcarbamate)Related Search:
1of4