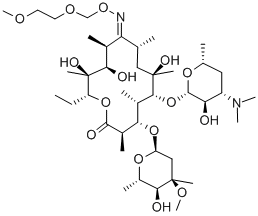
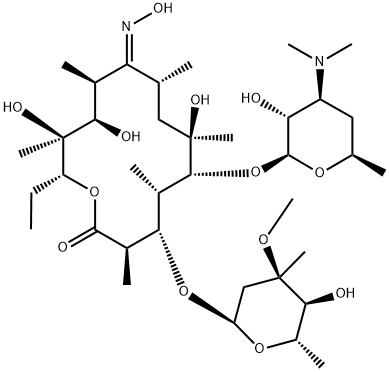
Roxithromycin
- CAS No.
- 80214-83-1
- Chemical Name:
- Roxithromycin
- Synonyms
- Roxid;Rulid;Roxithromycin Solution, 100ppm;Brilid;Overal;RU 965;Surlid;Assoral;Forilin;biaxsig
- CBNumber:
- CB1707342
- Molecular Formula:
- C41H76N2O15
- Molecular Weight:
- 837.05
- MDL Number:
- MFCD00214389
- MOL File:
- 80214-83-1.mol
- MSDS File:
- SDS
| Melting point | 111-118?C |
|---|---|
| alpha | D25 -77.5 ±2° (c = 0.45 in chloroform) |
| Boiling point | 864.7±75.0 °C(Predicted) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| Flash point | >110°(230°F) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | chloroform: soluble10mg/mL |
| pka | pKa 9.27±0.01(H2O t = 25.0 I = 0.15 (KCl)) (Uncertain) |
| form | powder |
| color | White to off-white |
| Water Solubility | Soluble in water to 18µg/ml |
| Merck | 14,8276 |
| InChIKey | RXZBMPWDPOLZGW-HEWSMUCTSA-N |
| CAS DataBase Reference | 80214-83-1 |
| FDA UNII | 21KOF230FA |
| ATC code | J01FA06 |
| EPA Substance Registry System | Erythromycin, 9-[O-[(2-methoxyethoxy)methyl]oxime], (9E)- (80214-83-1) |
Roxithromycin price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | R4393 | Roxithromycin ≥90% (HPLC) | 80214-83-1 | 1g | $43.8 | 2023-01-07 | Buy |
| Sigma-Aldrich | R4393 | Roxithromycin ≥90% (HPLC) | 80214-83-1 | 10g | $182 | 2023-01-07 | Buy |
| Alfa Aesar | J66213 | Roxithromycin, 97% | 80214-83-1 | 5g | $98 | 2024-03-01 | Buy |
| Alfa Aesar | J66213 | Roxithromycin, 97% | 80214-83-1 | 10g | $165 | 2024-03-01 | Buy |
| Cayman Chemical | 19465 | Roxithromycin ≥95% | 80214-83-1 | 1g | $33 | 2024-03-01 | Buy |
Roxithromycin Chemical Properties,Uses,Production
Pharmacological effects
Roxithromycin is a semi-synthetic 14-membered ring macrolide antibiotic. It has similar mechanism of action as erythromycin with its antibacterial effect in vivo being 1-4 times stronger than erythromycin. Its effect against Gram-positive bacteria is slightly worse than erythromycin; the effect against Legionella pneumophila is somehow stronger than erythromycin; for Chlamydia pneumoniae, Mycoplasma pneumoniae and Ureaplasma urealyticum, it has similar or slightly stronger antimicrobial effect compared with erythromycin.
This product can penetrate through the bacterial cell membrane, having reversible binding to bacterial ribosomes 50S subunit at close to the donor ("P" position), blocking the transfer of ribonucleic acid binding to the "P" position as well as blocking the transfer of the peptide chain from the acceptor position ("A" position) to "P" position, thus leading to the inhibition of bacterial protein synthesis and playing an antibacterial effect. It is characterized by faster access to macrophages, alveolar cells and neutrophils. In vitro studies have shown that roxithromycin has broad antibacterial spectrum with similar effect as erythromycin against a variety of Streptococcus (including A, B, C-type streptococcus and Streptococcus pneumoniae, but except G-type and enterococci), Streptococcus pneumoniae, Staphylococcus aureus, Staphylococcus aureus (except MRSA), Staphylococcus epidermidis, Legionella pneumophila, Haemophilus ducreyi, Chlamydia trachomatis, Mycoplasma pneumoniae and oral or vaginal anaerobic bacteria. Neisseria catarrhalis is highly sensitive to roxithromycin. It also has inhibitory effects on Campylobacter, Bordetella pertussis, Haemophilus influenzae, Mycobacterium tuberculosis and Diphtheria bacilli but has no antibacterial activity against other gram-negative bacteria. The in vivo antibacterial activity of this product was significantly stronger than that of erythromycin. It also has significant effect against chlamydia, mycoplasma, ureaplasma urealyticum, Treponema pallidum and Legionella infection. It also has relatively weak effect on the helicobacter, Neisseria gonorrhoeae, meningococcal and Bordetella pertussis. Some bacteria have cross-resistance to erythromycin and this product.
Indications
This product is applicable to the purulent Streptococcus caused pharyngitis and tonsillitis, susceptible bacteria-caused sinusitis, otitis media, acute bronchitis, acute exacerbation of chronic bronchitis, Mycoplasma pneumoniae or Chlamydia pneumoniae caused pneumonia; Chlamydia trachomatis caused urinary reproductive system infections (urethritis and cervicitis); sensitive bacteria caused infection of skin soft tissue and other parts of the body; it can also used for non-specific and gonococcal genital infection, Legionella caused infection and Pediatric Infections.
Roxithromycin is characterized by acid resistance and excellent oral absorption with T1/2 being 12 to 14 hours which is 6 times higher than erythromycin. It has wide distribution in the body and is able to reach high concentration in both the upper and lower respiratory tract, reproductive tract and skin and maintain for a long time. Most of the metabolism occurs in vivo.
Roxithromycin clinical evaluation: the curing rate of chlamydia urethritis, cervicitis clinical and bacteriological was 93% to 97%; the efficacy against genital infection is similar to doxycycline; the cure rate for skin infection is 90% or more; the effectiveness for the treatment of respiratory infections is 83% and 50% while the effectiveness for the treatment of pneumonia and dental infections are 79% and 85%.
Chemical properties
It appears as White crystalline powder, odorless, bitter taste. It is easily soluble in ethanol or acetone, more easily soluble in methanol or ether but almost do not dissolve in water. [Α] D23-77.5 ° ± 2 ° (c = 0.45, chloroform).
Uses
1. It belongs to macrolide antibiotics, being a kind of erythromycin derivative with the antibacterial spectrum being similar as erythromycin but having a 6 times stronger effect on erythromycin with high bioavailability. It is used for the treatment of respiratory tract infection, such as pneumonia, acute bronchitis, acute infection of chronic bronchitis, atypical pneumonia, and genitourinary system infection, skin and soft tissue infections, having good therapeutic effect and tolerance with smaller adverse reactions than erythromycin.
2. As the new generation of macrolide antibiotics;
3. The product belongs to a new generation of macrolide antibiotics, mainly used for Gram-positive bacteria, anaerobes, chlamydia and mycoplasma. Its antibacterial activity in vitro is similar as erythromycin with its in vivo effect being 1-4 times stronger than erythromycin. Pharmacological effects and indications 1, it has strong bactericidal effect on Gram-negative bacteria and some Gram-negative bacteria (meningococcus, Neisseria gonorrhoeae, influenza bacilli, pertussis, Brucella) and spirochetes, Mycoplasma pneumoniae and Rickettsia. Legionella is sensitive to it; its effect against mycoplasma and chlamydia is further enhanced.
4. 2, antibacterial mechanism: it can bind to the bacterial ribosome 50S subunit, through blocking the peptidyl transfer and MRNA displacement, further inhibiting the protein synthesis. 3, this product has excellent oral absorption and quick efficacy with stronger bioavailability and plasma concentrations than erythromycin. According to reports, oral administration of 150 mg can lead to a peak plasma concentration of up to 6.6~7.9 ug/ml while the value for erythromycin under the same conditions is only 0.2~0.4ug/ml; roxithromycin is mainly distributed in body fluids and tissues, with high and durable tissue concentration and cell concentration. Moreover, it has a half-life of up to 12 h while the value for erythromycin is only 1.5~2.0h. 4, in clinical medicine, it is the first-choice drug for the treatment of drug-resistance Staphylococcus aureus and hemolytic streptococcus. In veterinary clinical practice, it is used for the treatment of penicillin-resistant staphylococcus aureus caused severe infection, pneumonia, uterine inflammation and poultry chronic respiratory disease. 5, the goods are mainly used for the treatment of livestock and poultry chronic respiratory disease with good efficacy in the treatment of livestock and poultry staphylococcus and streptococcal disease, chlamydia, spirocheal disease (swine dysentery), infectious pleurisy and rhinitis. 6, indications: avian neck swelling, local heat, pain, difficulty breathing, mouth and nose flow white foam, mucous membranes and skin cyanosis and other symptoms.
Production method
Take erythromycin as raw materials for reaction with hydroxylamine hydrochloride in triethylamine and methanol, generating oxime, followed by reaction with methoxy ethoxymethyl chloride in acetone to derive roxithromycin.
Description
Roxithromycin, an oxime ether derivative of erythromycin, is a new macrolide antibiotic of high potency and long duration. Its in v i m activity spectrum is similar to that of erythromycin, but is superior to the latter against Legionella, Mycoplasma and Chlamydia.
Chemical Properties
White Solid
Originator
Roussel (France)
Uses
anesthetic (local)
Uses
Semisynthetic erythromycin derivative. Antibacterial
Uses
Roxithromycin was one of the new generation erythromycins introduced in the 1980s. Improved acid stability was achieved by converting the 9-keto group to the more stable oxime and alkylation of the oxime to provide the methoxyethoxymethyl ether oxime. In vivo, roxithromycin exhibits higher tissue levels and a longer half-life while being slightly less potent than erythromycin in vitro.
Definition
ChEBI: Roxithromycin is semisynthetic derivative of erythromycin A. It has a role as an antibacterial drug. It is an erythromycin derivative, a macrolide and a semisynthetic derivative. It is functionally related to an erythromycin A.
brand name
Rulide (Hoechst-Roussel).
Antimicrobial activity
Activity against common pathogens is comparable to that of erythromycin. It is active against L. monocytogenes, C. jejuni, H. ducreyi, G. vaginalis, Bord. pertussis, C. diphtheriae, B. burgdorferi, H. pylori, the M. avium complex, Chlamydia spp., and U. urealyticum.
Pharmaceutical Applications
A semisynthetic derivative of erythromycin A formulated for oral use.
Pharmacokinetics
Oral absorption:50–55%
Cmax 150 mg oral : 7.9 mg/L after 1.9 h
300 mg oral :10.8 mg/L after 1.5 h
Plasma half-life :10.5–11.9 h
Plasma protein binding :c. 90%
absorption and metabolism
Absorption is not affected by food. Oral administration with antacids or H2-receptor antagonists does not significantly affect bioavailability. In a direct comparison, the area under the time–concentration curve (AUC) produced by a 150 mg dose was 16 times greater than that produced by 250 mg erythromycin A. Behavior in children is broadly similar to that in adults, repeated doses of 2.5 mg/kg producing age-independent mean peak plasma concentrations around 10 mg/L at 1–2 h, but the apparent elimination half-life was longer (approximately 20 h).
It is saturably bound to α-acid glycoprotein in plasma. The plasma clearance appears to be dose dependent or plasma concentration dependent.
DistributionIt is widely distributed, but does not reach the CSF. Concentrations close to the simultaneous serum level have been found in tonsillar, lung, prostatic and other tissues. It achieves high levels in skin.
Distribution
It is widely distributed, but does not reach the CSF. Concentrations close to the simultaneous serum level have been found in tonsillar, lung, prostatic and other tissues. It achieves high levels in skin.
Metabolism and excretion
Less than 5% of the administered dose is eliminated as degradation products. Rather more than half the dose appears in the feces and only 7–10% (including metabolites) in the urine: up to 15% is eliminated via the lungs. Renal clearance increased in volunteers as the dose was raised from 150 to 450 mg,
and is decreased in elderly subjects. In patients in whom the creatinine clearance was <10 mL/min, the apparent elimination half-life rose to around 15.5 h and total body clearance was significantly reduced. The apparent elimination half-life was somewhat increased in patients with hepatic cirrhosis.
Side effects
It is generally well tolerated, adverse effects being described in 3–4% of patients, mostly gastrointestinal disturbance (abdominal pain, nausea and diarrhea). Headache, weakness, dizziness, rash and reversible changes in liver function tests and increased eosinophils and platelets have also been described.
Roxithromycin Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Henan Suikang Pharmaceutical Co.,Ltd. | +86-18239973690 +86-18239973690 | sales@suikangpharm.com | China | 311 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 444 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9206 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
View Lastest Price from Roxithromycin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-28 | Roxithromycin
80214-83-1
|
US $0.00-0.00 / kg | 1kg | 99% | 20tons | Sinoway Industrial co., ltd. | |
 |
2024-11-28 | Roxithromycin
80214-83-1
|
US $0.00 / kg | 25kg | 99% | 1tons | Henan Suikang Pharmaceutical Co.,Ltd. | |
 |
2024-11-27 | Roxithromycin
80214-83-1
|
US $0.00 / Kg/Drum | 25KG | 96.0%~102.0%; EP9.0/CP2015 | 10tons/month | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- Roxithromycin
80214-83-1
- US $0.00-0.00 / kg
- 99%
- Sinoway Industrial co., ltd.
-

- Roxithromycin
80214-83-1
- US $0.00 / kg
- 99%
- Henan Suikang Pharmaceutical Co.,Ltd.
-

- Roxithromycin
80214-83-1
- US $0.00 / Kg/Drum
- 96.0%~102.0%; EP9.0/CP2015
- WUHAN FORTUNA CHEMICAL CO., LTD
80214-83-1(Roxithromycin)Related Search:
1of4