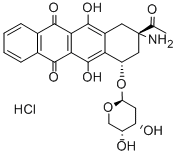
SM 5887
- CAS No.
- 110311-30-3
- Chemical Name:
- SM 5887
- Synonyms
- Calsed;SM 5887;AMR hydrochloride;Amrubicin hydrochloride;BHMLHEQFWVQAJS-IERVSNJOSA-N;(1S,3S)-3-Acetyl-3-amino-5,12-dihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydro-1-tetracenyl2-deoxy-β-D-erythro-pentopyranosidehydrochloride(1:1);(7S,9S)-9-Acetyl-9-amino-7-(2-deoxy-β-D-erythro-pentopyranosyloxy)-6,11-dihydroxy-5,7,8,9,10,12-hexahydro-5,12-naphthacenedione·hydrochloride
- CBNumber:
- CB21374151
- Molecular Formula:
- C25H25NO9.ClH
- Molecular Weight:
- 519.931
- MDL Number:
- MOL File:
- 110311-30-3.mol
| Melting point | 145-151° |
|---|---|
| FDA UNII | EUL6MP8FZW |
| NCI Drug Dictionary | amrubicin hydrochloride |
SM 5887 price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | API0004795 | SM 5887 95.00% | 110311-30-3 | 1MG | $329.7 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0004795 | SM 5887 95.00% | 110311-30-3 | 5MG | $504.74 | 2021-12-16 | Buy |
| Crysdot | CD31004574 | Amrubicinhydrochloride 98+% | 110311-30-3 | 5mg | $290 | 2021-12-16 | Buy |
SM 5887 Chemical Properties,Uses,Production
Description
small and small-cell lung cancers. Amrubicin, a completely synthetic anthracycline derivative, mediates its growth inhibitory action via topoisomerase II inhibition. It is activated in viva by the formation of its 13-OH metabolite, amrubicinol, via reaction with carbonyl reductase. In contrast to doxorubicin and daunorubicin whose metabolites are inactive in the blood, amrubicinol is 10-100 fold more cytotoxic than amrubicin. In phase II clinical trials, amrubicin showed antitumor activity against non-small-cell lung cancers (response rate exceeding 20%) and against untreated extensive stage small-cell-lung cancers (response rate 78.8%). Amrubicin demonstrated a smaller distribution-volume, a shorter half-life in mice and also less chronic cardiotoxicity in preclinical studies compared to doxorubicin. Amrubicin was generally well tolerated with major adverse events being anaemia, leucopenia, thrombocytopenia and neutropenia.
Originator
Sumitomo (Japan)
brand name
Calsed
SM 5887 Preparation Products And Raw materials
Raw materials
Preparation Products
SM 5887 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10991 | 58 |
| MedChemexpress LLC | 021-58955995 | sales@medchemexpress.cn | United States | 4863 | 58 |
| BOC Sciences | 16314854226 | info@bocsci.com | United States | 9926 | 65 |
| Musechem | +1-800-259-7612 | info@musechem.com | United States | 4662 | 60 |
| Shenzhen Botel Biotechnology Co. Ltd. | 0755-22202135 13316968096 | 1979313431@qq.com | China | 8618 | 58 |
| Beijing xinyanhui pharmaceutical research and development co., LTD | 13969155946 | 1461866103@qq.com | China | 16111 | 58 |
| Absin Bioscience Inc. | 021-38015121 15000105423 | wulan@absin.cn | China | 24734 | 58 |
| Hubei Xinyang Medical Technology Co., Ltd | 15347293736 15347293736 | 2853117764@qq.com | China | 7843 | 58 |
| Nantong QuanYi Biotechnology Co., Ltd | 0513-66337626 18051384581 | sales@chemhifuture.com | China | 4608 | 58 |
| Jiangsu Aikon Biopharmaceutical R&D co.,Ltd. | 025-66113011 18112977050 | cb5@aikonchem.com | China | 10529 | 58 |