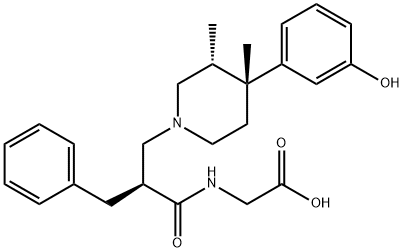
Alvimopan
- CAS No.
- 156053-89-3
- Chemical Name:
- Alvimopan
- Synonyms
- Ly246736;Alvimopan;Unii-Q153V49p3z;Alvimopan, ≥98%;Alvimopan anhydrous;Alvimopan USP/EP/BP;AlviMopan (ADL 8-2698);170098-38-1 (Dihydrate);trans-3,4-Dimethyl-4-(3-hydroxyphenyl) piperidine;((S)-2-benzyl-3-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)propanoyl)glycine
- CBNumber:
- CB21474721
- Molecular Formula:
- C25H32N2O4
- Molecular Weight:
- 424.53
- MDL Number:
- MFCD25970564
- MOL File:
- 156053-89-3.mol
- MSDS File:
- SDS
| Melting point | 200-204°C |
|---|---|
| Boiling point | 684.1±55.0 °C(Predicted) |
| Density | 1.166±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Sonicated, Heated) |
| pka | 3.39±0.10(Predicted) |
| form | Solid |
| color | White |
| FDA UNII | Q153V49P3Z |
| NCI Drug Dictionary | alvimopan |
| ATC code | A06AH02 |
Alvimopan price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 23902 | Alvimopan ≥98% | 156053-89-3 | 5mg | $97 | 2024-03-01 | Buy |
| Cayman Chemical | 23902 | Alvimopan ≥98% | 156053-89-3 | 10mg | $175 | 2024-03-01 | Buy |
| Cayman Chemical | 23902 | Alvimopan ≥98% | 156053-89-3 | 25mg | $410 | 2024-03-01 | Buy |
| Cayman Chemical | 23902 | Alvimopan ≥98% | 156053-89-3 | 100mg | $1184 | 2024-03-01 | Buy |
| TRC | A575800 | Alvimopan | 156053-89-3 | 100mg | $1190 | 2021-12-16 | Buy |
Alvimopan Chemical Properties,Uses,Production
Outline
Alvimopan has its chemical name being 2-[[(2S)-2-[[(3R, 4R)-4-(3-hydroxyphenyl)-3, 4-dimethyl-piperidin-1-yl] methyl]-3-phenyl-propionyl] amino] acetic acid dihydrate. It is a highly selective peripheral opioid antagonist which was jointly developed by the British company GlaxoSmithKline (GSK) and the United States Adolor company. In May 2008, it was approved by the US FDA for entering into market for applying to promote the recovering of the gastrointestinal function of patients who have been subject to colon or small intestine stage I resection anastomosis. Since the product can selectively inhibit the gastrointestinal opioid receptors without reducing the central analgesic effect of opioid agonists, therefore it does not affect the analgesic effect of systemic administration with safety and well-tolerance.
Preparation
1. Take 3-[(3R, 4R)-3,4-dimethyl-4-yl] phenol and 2-benzyl ethyl acrylate as raw material, go through Michael addition reaction to generate 2-benzyl-3-[(3R, 4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-piperidin-1-yl] ethyl propanoate, and then further go through hydrolysis, condensation, chiral column preparative separations and hydrolysis to obtain the alvimopan.
The products of each of the above steps need to be purified by column chromatography; in addition, about 50% of the optical isomers from the Michael addition reaction are required to be removed through chiral preparative separation in the condensation step. However, the operation is complicated and is not suitable for industrial production.
2. Use 3-[(3R, 4R)-3,4-dimethyl-4-yl] phenol, followed by the addition of methyl acrylate and benzyl bromide substitution in the presence of strong base lithium diisopropylamide (LDA) and salt formation to obtain (S)-2-benzyl-3-[(3R, 4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-piperidin-1-yl] propionic acid ethyl ester hydrochloride,3 hydrolyze to obtain (S)-2-benzyl-3-[(3R, 4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-piperidin-1-yl ] propionic acid monohydrate, then have condensation reaction with glycine isobutyl p-toluenesulfonate to obtain 2-[[(2S)-2-[[(3R, 4R)-4-(3-hydroxyphenyl)-3, 4-dimethylpiperidines-1-yl] methyl]-3-phenyl-propionyl]-amino] isobutyl acetate with hydrolysis generating alvimopan.
Mechanism
There are three opioid receptors μ, κ, and δ in the intestine reflection in regulating the gastrointestinal function. μ opioid receptor is the major receptor on gastrointestinal function and intestinal transit. It, through various kinds of signaling pathways including K + ion channels, membrane hyper-polarization, inhibiting Ca2 + ion channels and reducing the amount of cyclic AMP (cAMP), can achieve the cytosis effect of myenteric plexus cells. Opioid-class drugs binds to the intestinal opioid receptors, making bowel movements be slow, increase the muscle tension of the intestinal smooth muscle and non-peristaltic contractions. Surgical trauma may also stimulate the secretion of the endogenous opioid peptides, bind with the gastrointestinal μ-opioid receptor for inhibiting activity of the gastrointestinal tract. Exogenous and endogenous factors can stimulate the μ-opioid receptor activation, leading to gastrointestinal dysfunction. This product belongs to outer peripheral μ opioid receptor antagonist and can optionally bind with the μ-opioid receptors in the wall of the gastrointestinal, inhibit the stimulation of receptor activation on the mesenteric transfer activity. At the same time, the goods has no biological affinity to other non-opioid receptors such as adrenergic receptors (α1, α2, β), dopamine receptors (D1, D2), benzodiazepine receptors, serotonin receptors, histamine receptors (H1) and γ-amino butyric acid, and muscarinic receptor.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Pharmacodynamics
Pharmacokinetics: the bioavailability of oral administration of alvimopan is extremely poor, only 6% with the vast majority of the drug being present in the gastrointestinal tract for ensuring that drugs exert its role in local site. Human, after oral administration of 12 mg of alvimopan, has a small fraction get absorption with the Cmax being 14ng • ml-1, tmax being 1.5~3.0h and t1/2 being 1.3h. After absorption of the drug, it is rapidly removed with the drug being impermeable to blood-brain barrier. Drugs, through the metabolism of intestinal bacteria, are partially metabolized. However, the liver dose not to participate in drug metabolism and the original drug and its metabolites are excreted from the faeces.
Pharmacological effects
Using in vivo radio-ligand method, it has found that the product has a higher affinity to μ receptor affinity than δ receptor and κ receptors (Ki is respectively 0.77, 4.4 and 40 nmol • L-1). The radio-labeled product was found to have a high affinity to the cloned human μ opioid receptor with the KD value being 0.35nmol • L-1. This product can inhibit μ opioid receptors and bind to [3 5S]-GTPγS. The μ opioid receptors can reduce the amount of cAMP and the product has antagonistic effect. Compare the binding of [3H] naloxone or [3H] Morphine with that of the [3H] of this product with the human μ opioid receptor, [3H] of this product has a low dissociation rate, indicating a higher affinity for the receptor.
This product has been subject to the evaluation of the antagonist effect on peripheral and central μ opioid receptor in animal models. Studies have shown that this product has long-lasting inhibitory effect on the mice gastrointestinal dysfunction induced by morphine. The product has inhibitory effect on the analgesic effect of morphine only at high doses with being able to penetrate the blood brain barrier only at very high plasma concentration. The results have shown that the action of this product on the outer peripheral receptors is 200 times of that on central receptors 200 times, indicating that it has major antagonist effect on peripheral μ opioid receptor. Using charcoal mouse gavage study for evaluation of the pharmacological effect of this product, it has shown that it has an outer periphery-antagonist effect without central antagonist effect with certain therapeutic effect on the morphine-induced constipation of more than 8 h. Using morphine induced withdrawal jumping behavior for evaluation, the product does not alter the analgesic effect of morphine. This product is administered orally with a very low bioavailability which is only 6% for human. This product is mainly presented in the form of amphiphilic molecules so that the characteristic of this molecule is to prevent absorption in the gastrointestinal tract or penetrate through the blood-brain barrier to the central nervous system.
Drug Interactions
In vitro studies have shown that this product and its metabolites have no inhibitory effect on CYP1A2, CYP2C9, CYP2C19, CYP3A4, CYP2D6 and CYP2E1 while having no induction effect on CYP1A2, CYP2B6, CYP2C9, CYP2C19 and CYP3A4. Theoretically, there is no effect for drug of CYP enzyme inhibition or induction when being used in combination therapy with the product, but there is no clinical study data supporting this at present. This product and its metabolites are the substrate of the p-glycoprotein. There are still no clinical studies regarding to the combination medication between this product with potent inhibitor of p-glycoprotein (e.g. verapamil, cyclosporine, itraconazole, quinine, diltiazem, bepridil). Population pharmacokinetic analysis has showed that combined application of acid blockers or antibiotics does not affect the pharmacokinetic parameters of the product. This plasma concentration of this product metabolite is relative low and the metabolite has no efficacy, therefore it has no effect. Clinical upon intravenous infusion of morphine, combination with this product has no impact on the pharmacokinetic parameter on the morphine and its metabolites morphine-6 glucuronide.
Clinical application
1. It can be used for the treatment of post-operative ileus;
2. It can be used to treat the opioid-induced bowel dysfunction.
Side effects
The most common adverse reactions are nausea, vomiting, and hypotension.
Uses
flavenoid cytoprotective agent for the treatment of mucosal lesions
Uses
A peripheral μ-opioid receptor antagonist. Gastroprokinetic.
Definition
ChEBI: Alvimopan is a peptide.
Alvimopan Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29882 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| Shanghai Yingrui Biopharma Co.,Ltd | 21-33585366 | export01@shyrchem.com | CHINA | 1319 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29811 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| Qiuxian Baitai New Material Co., LTD | +8618330912755 | sale2@hbyalin.com | China | 1658 | 58 |
View Lastest Price from Alvimopan manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Alvimopan
156053-89-3
|
US $0.00 / Kg/Bag | 1KG | 99%min | 1000kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Alvimopan
156053-89-3
|
US $45.00-90.00 / mg | 99.97% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Alvimopan
156053-89-3
|
US $45.00-90.00 / mg | 99.97% | 10g | TargetMol Chemicals Inc. |