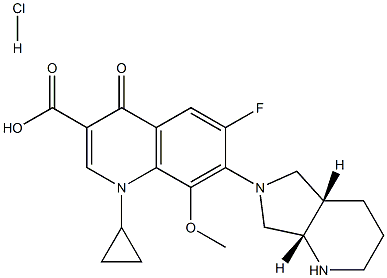
Moxifloxacin hydrochloride
- CAS No.
- 186826-86-8
- Chemical Name:
- Moxifloxacin hydrochloride
- Synonyms
- MOXIFLOXACIN HCL;Avelox;AVALOX;Moxifloxacin hydrochloride CRS;1-cyclopropyl-6-fluoro-8-methoxy-7-((4aS,7aS)-octahydropyrrolo[3,4-b]pyridin-6-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride;ACTIRA;Actura;MoxifL;PROFLOX;BAY-12-8039
- CBNumber:
- CB3191433
- Molecular Formula:
- C21H25ClFN3O4
- Molecular Weight:
- 437.8923032
- MDL Number:
- MFCD00949117
- MOL File:
- 186826-86-8.mol
- MSDS File:
- SDS
| Melting point | Slightly yellow to yellow crystalline powder, mp 324-325° |
|---|---|
| alpha | 25D -256° (c = 0.5 in water) |
| Boiling point | 636℃ |
| RTECS | VB1983750 |
| Flash point | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble5mg/mL, clear (warmed) |
| form | powder |
| color | white to beige |
| Water Solubility | Sparingly soluble in water. Soluble in DMSO |
| BRN | 8377447 |
| InChIKey | IDIIJJHBXUESQI-DFIJPDEKSA-N |
| SMILES | N1(C2CC2)C2=C(C=C(F)C(N3C[C@]4([H])CCCN[C@]4([H])C3)=C2OC)C(=O)C(C(O)=O)=C1.[H]Cl |&1:12,18,r| |
| CAS DataBase Reference | 186826-86-8(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Avelox; moxifloxacin hydrochloride |
| FDA UNII | C53598599T |
| NCI Drug Dictionary | Avelox |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H319-H412 | |||||||||
| Precautionary statements | P264-P270-P273-P280-P301+P312-P305+P351+P338 | |||||||||
| WGK Germany | 2 | |||||||||
| HazardClass | IRRITANT | |||||||||
| NFPA 704 |
|
Moxifloxacin hydrochloride price More Price(59)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR1542 | Moxifloxacin hydrochloride Pharmaceutical Secondary Standard; Certified Reference Material | 186826-86-8 | 1g | $112 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1448606 | Moxifloxacin hydrochloride United States Pharmacopeia (USP) Reference Standard | 186826-86-8 | 200mg | $1160 | 2024-03-01 | Buy |
| Sigma-Aldrich | 32477 | Moxifloxacin hydrochloride VETRANAL?, analytical standard | 186826-86-8 | 50MG | $335 | 2022-05-15 | Buy |
| Alfa Aesar | J66626 | Moxifloxacin hydrochloride, 97% | 186826-86-8 | 100mg | $180 | 2024-03-01 | Buy |
| Alfa Aesar | J66626 | Moxifloxacin hydrochloride, 97% | 186826-86-8 | 500mg | $776 | 2024-03-01 | Buy |
Moxifloxacin hydrochloride Chemical Properties,Uses,Production
Indications and Usage
Moxifloxacin Hydrochloride is a fluoroquinolone antibiotic developed by Bayer Pharmaceuticals (Germany.) It can be used to treat community-acquired pneumonia caused by Staphylococcus aureus, baccilus, pneumococcus, mucositis Moraxella, and Klebsiella pneumoniae, acute chronic bronchitis attacks, and acute sinusitis. For the treatment of adult bacterial lung infections, paranasal sinus, skin, and abdominal cavity. Also used to treat community-acquired pneumonia, chronic bronchitis, urogenital infection, and acute sinusitis.
Mechanisms of Action
Its active mechanisms and in vitro antibacterial spectrums are similar to those of other fluoroquinolones, but its profile towards gram-positive and anaerobic bacteria is similar to that of trovafloxacin, better than some older drugs. Compared with other fluoroquinolones, few gram-positive bacteria are resistant to Moxifloxacin Hydrochloride, or the spread of resistance is very slow. Gram-negative and enterococci strains with cross resistance to other fluoroquinolones have been found. It is effective at least against Staphylococcus aureus strains grlA, grlB, gyrA and gyrBcan, and 0.5-2 mg/L can inhibit Ciprofloxacin resistant Staphylococcus aureus, from large MIC to small Ciprofloxacin, Ofloxacin, Levofloxacin, Sparfloxacin, and Moxifloxacin.
Pharmacokinetics
After 45 healthy volunteers were orally administered single doses of 50-800 mg, their peak plasma concentration and area under the curve (AUC) increased linearly with dosage. Recommended oral dose is 400 mg, average Cmax is 2.5mg/L, peak time (tmax) is 1.5 hours, and AUC is 26.9 mg•h/L. For healthy volunteers who took 400 mg per day orally for 10 days, Cmax was 4.52 mg/L, 1.59 accumulated over 10 days. Oral bioavailability is 89%, apparent distribution volume 3.55 L/kg, plasma protein binding rate 48%. For 400 mg intravenously, Cmaxis 3.62 mg/L. AUC 34.6 mg•h/L. 24 hours after 13 healthy volunteers were given a single oral dose or intravenous injection of 400 mg, skin blister fluid concentration was twice that of serum, suggesting easier penetration of interstitial tissue. One hour after 18 patients ingested a single oral dose of 400 mg before undergoing bronchoscopy, bronchial epithelial cell fluid and bronchial biopsy tissue concentrations were 24.4 and 5.5 mg / L respectively, greater than the plasma concentration after 12 hours. An extremely high concentration (113.6 mg/L) was reached in macrophages. After 34 patients with chronic sinusitis received 5 oral doses of 400 mg, concentration in maxillary sinus mucosa exceeded blood plasma concentration. Three hours after the last dose, blood concentration peaked at 7.47 mg/kg; after 36 hours it was 1.25 mg/kg,suggesting a post-dosage effect.
After healthy volunteers received an oral dose of 400 mg, total clearance rate and renal clearance rates were 14.9 and 3.03 L per hour respectively. The drug apparently does not undergo P450 metabolism. Metabolized in vitro into N-sulfate and acyl glucuronide, metabolites inactive. After healthy volunteers took 400 mg/d orally, the average elimination half-life (t1/2β) during the first day was 9.3 hours, 11.95 hours over 10 days. Another study showed that the average t1/2β is about 10-16 hours. After a single oral dose or intravenous infusion of 400 mg, the urine reabsorption rates were 19%-20% and 22% respectively.
Adverse Effects
The adverse effects of this product are mostly mild and transient, and 3.8% of patients discontinued treatment as a result of adverse effects. The most common effects were nausea (7.2%) and diarrhea (5.7%). The incidence of dizziness was 2.8%. Healthy volunteers experienced no changes in vital signs, hematology, blood biochemistry, and ECG. Studies show that it is different from lomefloxacin and did not show any phototoxicity.
Warnings and Precautions
Similar to other fluoroquinones, bioavailability of 400 mg of Moxifloxacin Hydrochloride declined significantly after combination with antacids. AUC and cmax decreased 45% and 40% compared with when used alone, but Moxifloxacin Hydrochloride absorption was not significantly affected when taken 2 hours before or 4 hours after taking antacids. When taken with iron, absorption decreased significantly, with AUC and cmax was 39% and 59% lower, respectively. No interaction with theophylline, probenecid, ranitidine, or warfarine.
Adverse reactions
Side effects of this product are mostly mild and transient , 3.8% of the patient discontinued treatment due to adverse events . The most common adverse reactions are nausea (7.2%) and diarrhea (5.7%). Dizziness is 2.8%. In healthy volunteers, no changes in vital signs, hematology, blood biochemistry and electrocardiogram.
Studies have shown that the product is different from lomefloxacin , no drug-induced light toxicity.
Precautions
Similar to other fluoroquinolones , the product (400mg) in combination with antacids, bioavailability will fall significantly, AUC and cmax fall down 45% and 40% respectively when compared with alone, but using the moxifloxacin hydrochloride 2h before taking antacids or using antacids 4h after using this service, the absorption of the drug has no effect. If the product in combination with iron ,absorption rate decreases, AUC and cmax are reduced by 39% and 59%. The product has no interaction with theophylline, probenecid, ranitidine and warfarin .
Description
Moxifioxacin hydrochlodde is one of the two new fluoroquinolonecarboxylic acid antibiotics introduced in 1999 for the treatment of respiratory tract infections such as community-acquired pneumonia, acute exacerbations of bronchitis or acute sinusitis. Moxifloxacin can be synthesized through a 12 step sequence from the classical 4-quinolone-3-carboxylic acid template. Some advantages of Moxifloxacin over Ciprofloxacin, another of Bayer's launched quinolones, have been shown, i.e. an enhanced activity against Gram-positive bacteria (Streptococcus pneumoniae, Clostndium pneumoniae), a favorable pharmacokinetic profile (good tissue penetration and plasma concentrations above MICs) and a lack of phototoxicity (UVA irradiation).
Description
Moxifloxacin is a fluoroquinolone antibiotic. It is active against 390 clinical isolates of aerobic and anaerobic Gram-positive and Gram-negative bacteria (MIC90s = ≤0.25 μg/ml), as well as clinical isolates of methicillin-susceptible and -resistant S. aureus (MIC90s = 0.12 and 2 μg/ml, respectively). Moxifloxacin is an inhibitor of E. coli DNA gyrase that is selective for DNA gyrase over E. coli topoisomerase IV (IC50s = 0.51 and 38.8 mg/L, respectively, in cell-free assays). It prevents S. aureus- or P. aeruginosa-induced increases in bronchoalveolar lavage fluid (BALF) neutrophil infiltration and reduces S. aureus- or P. aeruginosa-induced increases in lung chemokine (C-X-C motif) ligand 1 (CXCL1) and IL-1β levels in mouse models of bacterial pneumonia when administered at a dose of 100 mg/kg twice per day for two days. Moxifloxacin (100 mg/kg) decreases the number of lung and spleen colony forming units (CFUs) in a mouse model of systemic M. tuberculosis infection. Formulations containing moxifloxacin have been used in the treatment of various bacterial infections.
Chemical Properties
Light yellow to yellow crystalline powder
Originator
Bayer (Germany)
Uses
Moxifloxacin Hydrochloride is an antibacterial agent that inhibits the activities of Topo II (DNA gyrase) and topoisomerase IV.
Uses
acetylcholinesterase inhibitor (reversible), cognitive enhancer
Definition
ChEBI: Moxifloxacin hydrochloride is a hydrochloride comprising equimolar amounts of moxifloxacin and hydrogen chloride. It has a role as an antibacterial drug. It contains a moxifloxacinium(1+).
Manufacturing Process
Manufacturing process for Moxifloxacin hydrochloride includes 3 steps: Synthesis of intermidate octahydropyrrolo[3,4-b]pyridine (2,8- diazabicyclo[4.3.0]nonane);Synthesis of intermidate 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4- oxo-3-quinolinecarboxylic acid;Syntesis of 1-cyclopropyl-7-(2,8-diazabicyclo[4.3.0]non-8-yl)-6-fluoro-1,4- dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid hydrochloride (moxifloxacin hydrochloride)
brand name
Avelox (Bayer); Vigamox (Alcon.
Therapeutic Function
Antibacterial
General Description
Moxifloxacin Hydrochloride is an anti-infectious compound that belongs to the class of fluoroquinolone antibiotics. It is a well-known broad-spectrum antibiotic prescription medicine effective against a variety of Gram-positive and Gram-negative bacteria including the anaerobic type. It is an S,S-isomer with two chiral centers. It works by preventing the activity of various crucial enzymes involved in the replication, transcription, recombination, and repair of deoxyribonucleic acid in the bacteria such as DNA gyrase (topoisomerase II), topoisomerase IV. It finds its application in the treatment of various ocular and respiratory tract infections.
Biochem/physiol Actions
Moxifloxacin has proved to be effective in treating sinusitis, community-acquired respiratory tract infection, pneumonia and acute exacerbations of chronic bronchitis. It has improved anti-gram positive activity compared to other fluoroquinolone such as ciprofloxacin and ofloxacin. Moxifloxacin is useful in treating skin infections that is of bacterial origin. It is known to have proper penetration into peripheral tissues and inflammatory fluids. Fluoroquinolones stabilize DNA strand breaks created by DNA gyrase and topoisomerase IV by binding to the enzyme-DNA complex. Compared to mammalian DNA gyrase, moxifloxacin has 100 times higher affinity for bacterial DNA gyrase. Moxifloxacin is an antibiotic and works against both Gram-positive and Gram-negative bacteria. Moxifloxacin is being investigated for the treatment of multidrug-resistant tuberculosis.
References
[1]. cruz la, hall r: enantiomeric purity assay of moxifloxacin hydrochloride by capillary electrophoresis. j pharm biomed anal 2005, 38(1):8-13.
[2]. kamruzzaman m, alam am, lee sh, ragupathy d, kim yh, park sr, kim sh: spectrofluorimetric study of the interaction between europium(iii) and moxifloxacin in micellar solution and its analytical application. spectrochim acta a mol biomol spectrosc 2012, 86:375-380.
[3]. culley cm, lacy mk, klutman n, edwards b: moxifloxacin: clinical efficacy and safety. am j health syst pharm 2001, 58(5):379-388.
[4]. turkes c, soyut h, beydemir s: human serum paraoxonase-1 (hpon1): in vitro inhibition effects of moxifloxacin hydrochloride, levofloxacin hemihidrate, cefepime hydrochloride, cefotaxime sodium and ceftizoxime sodium. j enzyme inhib med chem 2014:1-7.
Moxifloxacin hydrochloride Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zibo Wei Bin Import & Export Trade Co. Ltd. | +86-0533-2091136 +8613864437655 | ziboweibinmaoyi@163.com | China | 100 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12813 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 | allison@yan-xi.com | China | 5854 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5870 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 987 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20235 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 485 | 58 |
| Hebei Junhua Import and Export Co., LTD | +86-18503288844 +86-18503288844 | junhua1@hb-junhua.com | China | 977 | 58 |
Related articles
- Moxifloxacin Hydrochloride: A Comprehensive Overview
- Moxifloxacin hydrochloride stands out as a powerful antibacterial agent with broad-spectrum activity and versatile clinical ap....
- Dec 31,2024
View Lastest Price from Moxifloxacin hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-22 | Moxifloxacin hydrochloride
186826-86-8
|
US $0.00 / kg | 1kg | 98% | 1000 | Hebei Junhua Import and Export Co., LTD | |
 |
2025-04-22 | Moxifloxacin hydrochloride
186826-86-8
|
US $0.00 / Kg/Bag | 1KG | 98.0%~102.0%; EP9.0 | 2500kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-22 | Moxifloxacin hydrochloride
186826-86-8
|
US $0.00-0.00 / KG | 1KG | 99% up, DMF | 20 tons | Sinoway Industrial co., ltd. |
-

- Moxifloxacin hydrochloride
186826-86-8
- US $0.00 / kg
- 98%
- Hebei Junhua Import and Export Co., LTD
-

- Moxifloxacin hydrochloride
186826-86-8
- US $0.00 / Kg/Bag
- 98.0%~102.0%; EP9.0
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Moxifloxacin hydrochloride
186826-86-8
- US $0.00-0.00 / KG
- 99% up, DMF
- Sinoway Industrial co., ltd.
186826-86-8(Moxifloxacin hydrochloride)Related Search:
1of4