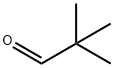
Pivaldehyde
- CAS No.
- 630-19-3
- Chemical Name:
- Pivaldehyde
- Synonyms
- PIVALALDEHYDE;PAL;TRIMETHYLACETALDEHYDE;2,2-DIMETHYLPROPANAL;Pivalic aldehyde;tert-Pentanal;propanal,2,2-dimethyl-;LRIT1;LRRC21;NSC 22043
- CBNumber:
- CB2174507
- Molecular Formula:
- C5H10O
- Molecular Weight:
- 86.13
- MDL Number:
- MFCD00006962
- MOL File:
- 630-19-3.mol
- MSDS File:
- SDS
| Melting point | 6 °C(lit.) |
|---|---|
| Boiling point | 74 °C730 mm Hg(lit.) |
| Density | 0.793 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | 4 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.793 |
| Water Solubility | Negligible |
| Sensitive | Air Sensitive |
| BRN | 506060 |
| Dielectric constant | 9.0500000000000007 |
| Stability | Air Sensitive, Volatile |
| InChIKey | FJJYHTVHBVXEEQ-UHFFFAOYSA-N |
| CAS DataBase Reference | 630-19-3(CAS DataBase Reference) |
| FDA UNII | SSC20260QW |
| NIST Chemistry Reference | Propanal, 2,2-dimethyl-(630-19-3) |
| EPA Substance Registry System | Propanal, 2,2-dimethyl- (630-19-3) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H315-H319-H335 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi,F+ | |||||||||
| Risk Statements | 11-37/38-43 | |||||||||
| Safety Statements | 16-23-33-24 | |||||||||
| RIDADR | UN 1989 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29121900 | |||||||||
| NFPA 704 |
|
Pivaldehyde price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T71501 | Trimethylacetaldehyde 96% | 630-19-3 | 5ml | $65.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | T71501 | Trimethylacetaldehyde 96% | 630-19-3 | 25ml | $240 | 2024-03-01 | Buy |
| TCI Chemical | P0847 | Pivalaldehyde >95.0%(GC) | 630-19-3 | 5mL | $50 | 2024-03-01 | Buy |
| TCI Chemical | P0847 | Pivalaldehyde >95.0%(GC) | 630-19-3 | 25mL | $146 | 2024-03-01 | Buy |
| Alfa Aesar | A15013 | Trimethylacetaldehyde, 95% | 630-19-3 | 5g | $67.65 | 2024-03-01 | Buy |
Pivaldehyde Chemical Properties,Uses,Production
Description
Trimethylacetaldehyde (also known as Pivaldehyde) is the trimethyl form of acetaldehyde. It is an aldehyde with a sterically bulky R group, the tertiary-butyl group being attached to the carbonyl, >C=O. It is a useful reagent in organic synthesis, pharmaceuticals, agrochemicals and dyestuff. It is useful in streoselective synthesis application as well as in aldol condensation reactions. As a derivative of acetaldehyde, trimethyl acetaldehyde can be used for the production of acetate. It can also be used as the precursor for manufacturing of pyridine derivatives, pentaerythritol, and crotonaldehyde.
Chemical Properties
Clear colorless liquid
Uses
Trimethylacetaldehyde is used as a stereoselective synthesis application. It is also used as a building block used in aldol condensation reactions. It is an important raw material and intermediate used in Organic Synthesis, Pharmaceuticals, Agrochemicals and Dyestuff.
Uses
Commonly used building block in aldol condensation reactions.
Definition
ChEBI: 2,2-dimethylpropanal is a member of the class of propanals that is propanal substituted by two methyl groups at position 2. It is a member of propanals and a 2-methyl-branched fatty aldehyde.
Application
Pivaldehyde is a hazardous by-product released from engine exhaust and is often used as a raw material for organic synthesis or as a reagent in chemical reactions. It can be copolymerised with aldehyde anions, such as acrolein, to prepare unsaturated polyacetals, or a TBPB initiated decarbonylative cascade cyclization of ortho-cyanoarylacrylamides with pivaldehyde leads to tert-butyl containing quinolone-2,4(1H,3H)-diones by formation of both C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds. As well as probing the absolute rate constants of its autoxidation, etc[1-3].
References
[1] A. LAPENA R. S J L Mateo. Anionic copolymerization of acrolein with aldehydes. III[J]. Journal of Polymer Science: Polymer Symposia, 1973, 42 1: 311-319. DOI:10.1002/polc.5070420133.
[2] CUI ZHANG. Decarbonylative cascade cyclization of ortho-cyanoarylacrylamides with pivaldehyde: Access to tert-butyl containing quinolone-2,4(1H,3H)-diones[J]. Tetrahedron, 2022. DOI:10.1016/j.tet.2021.132547.
[3] G. ZAIKOV K. I J Howard. Absolute rate constants for hydrocarbon autoxidation. XIII. Aldehydes: photo-oxidation, co-oxidation, and inhibition[J]. Canadian Journal of Chemistry, 1969. DOI:10.1139/V69-500.
[4] Ho, Tse Lok, et al. Pivaldehyde. Fieser and Fieser's Reagents for Organic Synthesis. John Wiley & Sons, Inc. 2006.
[5] https://en.wikipedia.org/wiki/Acetaldehyde#Uses
[6] https://en.wikipedia.org/wiki/Pivaldehyde
[7] https://www.alfa.com/en/catalog/A15013/
[8] GEORGE A. OLAH. Acid-Catalyzed Isomerization of Pivalaldehyde to Methyl Isopropyl Ketone via a Reactive Protosolvated Carboxonium Ion Intermediate?[J]. Journal of the American Chemical Society, 2001, 123 47: 11556-11561. DOI:10.1021/ja011253a.
[9] O. SUGIMOTO. Preparation and reaction of quinolinyl (or pyridinyl)phosphonium salts with base and pivalaldehyde[J]. Heterocycles, 2011, 56 1: 837-847. DOI:10.3987/COM-11-12154.
[10] SUNG MAN PARK Chan H K Yu Ran Lee. Conformational Structures of Neutral and Cationic Pivaldehyde Revealed by IR-Resonant VUV-MATI Mass Spectroscopy.[J]. International Journal of Molecular Sciences, 2022, 23 23. DOI:10.3390/ijms232314777.
Pivaldehyde Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Handan Huajun chemicals Co.,Ltd | +86-0310-8166573 +8618630058311 | sales@huajunchem.com | China | 30 | 58 |
| PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD | +86-13572219112 +86-15029287646 | info@pushanshiye.com | China | 165 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5876 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | sales@ichemie.com | China | 1015 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
| Hebei Junhua Import and Export Co., LTD | +86-18503288844 +86-18503288844 | junhua1@hb-junhua.com | China | 977 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| Zjartschem | +86-571 87238903 | jocelynpan@zjarts.com | CHINA | 988 | 58 |
Related articles
- Pivaldehyde: applications and safety
- Pivaldehyde's distinctive reactivity drives its diverse applications in organic synthesis and pharmaceutical development, desp....
- Nov 23,2023
- The use of pivaldehyde in organic synthesis
- Pivaldehyde is an example of an aldehyde with the tertiary-butyl group (with 3 methyl groups), attached to the carbonyl (C=O)....
- Dec 12,2019
View Lastest Price from Pivaldehyde manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-03-26 | Pivaldehyde
630-19-3
|
US $120.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2025-03-26 | Pivalaldehyde
630-19-3
|
US $190.00 / kg | 1kg | 96.0% | 500MT | Handan Huajun chemicals Co.,Ltd | |
 |
2025-03-26 | Pivaldehyde
630-19-3
|
US $150.00 / GREM | 10GREM | 99% | 1000 | Hebei Junhua Import and Export Co., LTD |
-

- Pivaldehyde
630-19-3
- US $120.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Pivalaldehyde
630-19-3
- US $190.00 / kg
- 96.0%
- Handan Huajun chemicals Co.,Ltd
-

- Pivaldehyde
630-19-3
- US $150.00 / GREM
- 99%
- Hebei Junhua Import and Export Co., LTD
630-19-3(Pivaldehyde)Related Search:
1of4