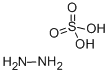Hydrazine sulfate
- CAS No.
- 10034-93-2
- Chemical Name:
- Hydrazine sulfate
- Synonyms
- HYDRAZINIUM SULFATE;HYDRAZINE SULPHATE;Diamine sulfate;HYDRAZINIUM SULPHATE;nsc-150014;siranhydrazinu;idrazinasolfato;Hydrazine Sulfat;diamidogensulfate;HYDRAZINE SULFATE
- CBNumber:
- CB2679815
- Molecular Formula:
- H6N2O4S
- Molecular Weight:
- 130.12
- MDL Number:
- MFCD00044873
- MOL File:
- 10034-93-2.mol
- MSDS File:
- SDS
| Melting point | 254 °C(lit.) |
|---|---|
| Density | 1,37 g/cm3 |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 30g/l |
| form | Crystalline Powder |
| Specific Gravity | 1.37 |
| color | White |
| PH | 1.5 (50g/l, H2O, 20℃) |
| Odor | Odorless |
| PH Range | 1.3 (0.2 M solution) |
| Water Solubility | 30 g/L (20 ºC) |
| Merck | 14,4772 |
| BRN | 8778859 |
| Stability | Stable. Incompatible with nitrites, strong oxidizing agents. |
| InChIKey | ZGCHATBSUIJLRL-UHFFFAOYSA-N |
| CAS DataBase Reference | 10034-93-2(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | hydrazine sulfate |
| FDA UNII | 1N369SAT01 |
| NCI Drug Dictionary | hydrazine sulfate |
| Proposition 65 List | Hydrazine sulfate |
| NIST Chemistry Reference | Hydrazine dihydrogen sulfate (salt)(10034-93-2) |
| EPA Substance Registry System | Hydrazine sulfate (1:1) (10034-93-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H314-H317-H350-H410 | |||||||||
| Precautionary statements | P260-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 45-23/24/25-43-50/53-35 | |||||||||
| Safety Statements | 53-45-60-61-36/37/39-26 | |||||||||
| RIDADR | UN 2923 8/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MV9625000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28251090 | |||||||||
| Toxicity | Hydrazine sulfate is moderately toxic. Symptoms of ingestion are paresthesia, somnolence, nausea, and vomiting. It also is an irritant to the eye. It is a confirmed carcinogen and an experimental teratogen. | |||||||||
| NFPA 704 |
|
Hydrazine sulfate price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 216046 | Hydrazine sulfate salt ACS reagent, ≥99.0% | 10034-93-2 | 5g | $61.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 216046 | Hydrazine sulfate salt ACS reagent, ≥99.0% | 10034-93-2 | 100g | $84.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04603 | Hydrazinium sulfate GR for analysis ACS,Reag. Ph Eur | 10034-93-2 | 100g | $94.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04603 | Hydrazinium sulfate GR for analysis ACS,Reag. Ph Eur | 10034-93-2 | 500g | $132 | 2024-03-01 | Buy |
| Alfa Aesar | A10792 | Hydrazine sulfate, 99+% | 10034-93-2 | 1kg | $152 | 2024-03-01 | Buy |
Hydrazine sulfate Chemical Properties,Uses,Production
Description
Hydrazine sulfate is salt which generated by hydrazine and sulfuric acid, pure product is colorless scaly crystal or rhombic. The molecular weight is 130.12. Formula is N2H4·H2SO4. Melting point is 254℃, when continues heating, it can decompose. The relative density is 1.37. It is slightly soluble in water, soluble in hot water (at 20℃ 2.87, at 25℃ 3.41, at 30℃ 3.89, at 40℃ 4.16, at 50 ℃ 7.0, at 60℃ 9.07, at 80℃ 14.4), aqueous solution is acidic , it is insoluble in alcohol and ether. It is very stable in air. It is susceptible to alkali and oxidizing agents, it is incompatible with bases, oxidants. It has strong reduction. Rat oral LD50601mg/kg, it is toxic, carcinogenic. The main purpose of hydrazine sulfate is used for determination the weight of nickel, cobalt, cadmium, and the purification of rare metals, the separation of tellurium and polonium, as well as the precipitation of chlorate, hypochlorite and carboxyl compound, it is also used for manufacturing isoniazid , nitrofurazone, 100 Health hydrazine, anhydrous hydrazine, etc., it is also used as reducing agent, insecticides and sterilization agents. Hydrazine sulfate is nutritional supplement in United States for fighting against anorexia, weight loss and other symptoms patients which caused by cancer.
Laboratory method for preparing hydrazine sulfate
1. Sodium hypochlorite is prepared by chlorine and sodium hydroxid, then reacts with ammonia to obtain hydrazine hydrate, and finally to obtain hydrazine by dehydration, and then reacts with sulfuric acid to obtain hydrazine sulfate products. Related chemical reaction equation is as follows:
2NH3 + NaClO → NH2Cl + NaOH
NH2Cl + NaOH + NH3 → N2H4 + NaCl + H2O
N2H4 + H2SO4 → N2H4 · H2SO4
2. When ammonia reacts with water, ammonium hydroxide can be obtained, and then by synthesis, hydrazine solution can be obtained, by evaporation, condensation, crystallization, and finally reacts with sulfuric acid to obtain hydrazine sulfate products.
Chemical properties
It is colorless scaly crystal or orthorhombic crystal. It is tasteless. It is slightly soluble in water, soluble in water, acidic aqueous solution. It is insoluble in alcohol.
Uses
1. It can be used as analytical reagent and reducing agent, it can also be used for the purification of rare metals.
2. It can be used as raw material for the manufacture of pharmaceutical. It can be used as raw material for azobisisobutyronitrile and other products in organic industry. It can be used as reducing agent on the plating. It can be used as insecticide, sterilizing agent in agriculture. It can be used as blowing agents in plastics and rubber.
3. It can be used for weight determination of nickel, cobalt and cadmium, purification of rare metals, reducing agents, organic synthesis, separation of polonium and tellurium, determination of hypochlorite, hypochlorous acid and carboxyl compounds, thymol turbidity is prepared in liver function tests. It can be used for isoniazid, nitrofurazone, 100 Health hydrazine, anhydrous hydrazine, pesticides and fungicides, for rocket fuel processing, anti-rust products, manufacture of ADC foaming agent. It is widely used in medicine, organic synthesis, pesticides, plastics, rubber and other industries.
The above information is edited by the chemicalbook of Wang Xiaodong.
Production method
Urea method: Urea, sodium hypochlorite, caustic soda, in the presence of potassium permanganate can react, by distillation, and then reacts with sulfuric acid for neutralization, the resultant passes through cooling and crystallization, filtration, drying to obtain hydrazine sulfate products.
NaOCI + NH2CONH2 + 2NaOH [KMnO4] → N2H4·H2O + NaCl + Na2CO3
N2H4·H2O + H2SO4 → N2H4·H2SO4 + H2O
Description
Hydrazine sulfate is a white or colorless, crys-talline powder; Freezing/Melting point = 254℃ (decompo-sition). HazardIdentification (based on NFPA-704 MRating System): Health 3, Flammability 1, Reactivity 1.Soluble in water.
Chemical Properties
colourless crystals or white powder
Physical properties
Colorless orthorhombic crystal; density 1.378 g/cm3; melts at 254°C; sparingly soluble in cold water 1.64% at 0°C and 3.41% at 25°C; more soluble inhot water; practically insoluble in alcohol (0.04% at 25°C).
Uses
Reagent for determination of C-terminal amino acidsHydrazine sulfate is used in analytical chemistry for the gravimetric estimation of nickel, cobalt and cadmium. It is also used in the refining of rare metals as well as in the separation of polonium from tellurium. It finds application in the synthesis of chemical intermediate and organic compounds. Further, it acts as an antioxidant in soldering flux for light metals and as a precursor to hydrazine.
Uses
Hydrazine sulfate is used in the gravimetric estimation of nickel, cobalt and cadmium; in the refining of rare metals; as an antioxidant in soldering flux for light metals; as a reducing agent in the analysis of minerals and slags; in separating polonium from tellurium; in tests for blood; for destroying fungi and molds; in the preparation of hydrazine hydrate; press ure stabilizer in cutting oils.
Uses
In the gravimetric estimation of nickel, cobalt and cadmium; in the refining of rare metals; as antioxidant in soldering flux for light metals; as reducing agent in the analysis of minerals and slags; in separating polonium from tellurium; in tests for blood; for destroying fungi and molds; in the preparation of hydrazine hydrate.
Preparation
Hydrazine sulfate may be synthesized from aqueous ammonia and sodium hypochlorite solution in a two-step process. In the first stage, aqueous solution of ammonia is boiled with a normal solution of sodium hypochlorite in the presence of 10% gelatin solution to yield hydrazine. In the second stage, the hydrazine solution is ice-cooled followed by slow addition of concentrated sulfuric acid (Adams, R., and B.K. Brown. 1964. In Organic Synthesis, Collective Volume I, ed. H. Gilman and A. H. Blatt, 2nd ed. pp 309-310, New York: John Wiley & Sons). The reaction steps are as follows:
2NH3 + NaOCl → NH2NH2 + H2O + NaCl
NH2NH2 + H2SO4 → NH2NH2 •H2SO4
General Description
Hydrazine sulfate salt is a potential anticachexia agent for treating cancer patients.
Hazard
A carcinogen (OSHA).
Potential Exposure
Used in analysis and refining of miner-als, rare metals, determination of arsenic in metals, as a .catalyst and an antioxidant, and in fungicides, germicides,and blood tests. Used as a catalyst for making acetatefibers.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irri gate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Asfirst aid for pulmonary edema, a doctor or authorized para-medic may consider administering a corticosteroid spray.Notetophysician:Treat formethemoglobinemia.Spectrophotometry may be required for precise determina-tion of levels of methemoglobin in urine. Pyridoxine(25 mg/kg) is an effective anticonvulsant for hydrazinepoisoning.
Carcinogenicity
Hydrazine and hydrazine sulfate are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals.
storage
(1) Color Code- Yellow Stripe (strong reducingagent): Reactivity Hazard; Store separately in an area iso-lated from flammables, combustibles, or other yellow-codedmaterials. (2) Color Code- White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.(3) Color Code- -Blue: Health Hazard/Poison: Store in asecure poison location. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045. Store intightly closed containers in a cool, well-ventilated areaaway from oxidizers, bases.
Shipping
Hydrazinesulfatemustbelabeled“CORROSIVE." It falls in Hazard Class 8 and PackingGroup III.
Purification Methods
Its solubility in H2O is 3% at room temperature, but is very soluble in hot H2O. It is a suspected carcinogen. [Adams & Brown Org Synth Coll Vol I 309 1941, Audrieth & Nickles Inorg Synth I 90 1939.]
Incompatibilities
A strong reducing agent. Reacts with oxi-dizers, bases.
Hydrazine sulfate Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5858 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18194 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 997 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 | admin@hbsaisier.cn | China | 1015 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 | admin@hblongbang.com | China | 941 | 58 |
Related articles
- What is Hydrazine sulfate?
- Hydrazine Sulfate is a salt of the cation hydrazinium and the anion bisulfate (hydrogen sulfate), with the formula [N2H5+] [HS....
- Jan 14,2020
View Lastest Price from Hydrazine sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-02 | Hydrazine sulfate
10034-93-2
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-01 | Hydrazine sulfate
10034-93-2
|
US $65.00-20.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-11-01 | Hydrazine sulfate
10034-93-2
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd |
-

- Hydrazine sulfate
10034-93-2
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Hydrazine sulfate
10034-93-2
- US $65.00-20.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Hydrazine sulfate
10034-93-2
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd