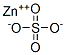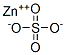Zinc sulphate
- CAS No.
- 7733-02-0
- Chemical Name:
- Zinc sulphate
- Synonyms
- ZINC SULFATE;ZnSO4;ZDDP;sulphate zinc;Zinc(II) sulfate;Zinc sulfate (ZnSO4);Sulfuric acid zinc salt;neozin;Z-Span;Bonazen
- CBNumber:
- CB2727299
- Molecular Formula:
- ZnSO4
- Molecular Weight:
- 161.45
- MDL Number:
- MFCD00011302
- MOL File:
- 7733-02-0.mol
- MSDS File:
- SDS
| Melting point | 100°C |
|---|---|
| Boiling point | 105°C (estimate) |
| Density | 1.31 g/mL at 20 °C |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: soluble |
| form | Liquid |
| color | Colorless |
| PH | 4.0±0.5 |
| Water Solubility | Soluble |
| λmax |
λ: 260 nm Amax: <0.02 λ: 280 nm Amax: <0.02 |
| Merck | 14,10159 |
| LogP | -1.031 (est) |
| Indirect Additives used in Food Contact Substances | ZINC SULFATE, ANHYDROUS |
| CAS DataBase Reference | 7733-02-0(CAS DataBase Reference) |
| FDA UNII | 0J6Z13X3WO |
| NIST Chemistry Reference | Zinc sulfate(7733-02-0) |
| EPA Substance Registry System | Zinc sulfate (7733-02-0) |
| UNSPSC Code | 12352302 |
| NACRES | NA.25 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS05,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H318-H411-H410-H319-H401 |
| Precautionary statements | P264-P280i-P337+P313-P273-P280-P305+P351+P338-P501 |
| Hazard Codes | Xn,N,Xi |
| Risk Statements | 52/53-50/53-41-22-51/53 |
| Safety Statements | 61-39-26-60 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| RTECS | ZH5260000 |
| TSCA | Yes |
| HS Code | 2833 29 20 |
| HazardClass | 9 |
| PackingGroup | III |
| Hazardous Substances Data | 7733-02-0(Hazardous Substances Data) |
Zinc sulphate price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 83265 | Zinc sulfate solution BioUltra, for molecular biology, 2.0?M in H2O | 7733-02-0 | 250ML | $56.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 83265 | Zinc sulfate solution BioUltra, for molecular biology, 2.0?M in H2O | 7733-02-0 | 1L | $189 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.09991 | Zinc sulfate solution for 1000 ml, c(ZnSO?) = 0.1 mol/l (0.1 N) Titrisol? | 7733-02-0 | 1amp | $61.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.08879 | Zinc sulfate solution c(ZnSO4) = 0.1 mol/l, Titripur?, reag. Ph. Eur. | 7733-02-0 | 1L | $62.3 | 2024-03-01 | Buy |
| Alfa Aesar | J60963 | Zinc sulfate 100mM aq. soln. | 7733-02-0 | 100ml | $60.7 | 2023-06-20 | Buy |
Zinc sulphate Chemical Properties,Uses,Production
Description
Zinc sulfate appears as colorless or white rhombic crystals or powder at room temperature. It has convergence property and is easily soluble in water with its aqueous solution being acidic. It is slightly soluble in ethanol and glycerol. Pure zinc sulfate can be stored in the air for a long time without turning yellow. It can lose water to become white powder when placed in dry air. There are various kinds of hydrates: in the range of 0-39 ° C, its stable hydrate balanced with aqueous phase is zinc sulfate heptahydrate; in the range of 39-60 ° C, it is hexahydrate zinc sulfate. At the range of 60-100 °C, it will become zinc sulfate monohydrate. When being heated to 280 °C, various kinds of hydrate will completely lose water with decomposition into zinc sulfate at 680 °C and further decomposition at above 750 ° C and finally decomposition into zinc oxide and sulfur trioxide at about 930 °C. ZnSO4 • 7H2O can form mixed crystal with MSO4 • 7H2O (M = Mg, Fe, Mn, Co, Ni) within a certain range. It is mainly used for the preparation of raw materials of pigment lithopone, zinc barium and other zinc compounds. It also has various kinds of applications such as animal nutrition upon zinc deficiency, animal feed additives, crop zinc fertilizer (trace element fertilizer), important materials of artificial fiber, electrolyte solution upon electrolytic production of zinc metal, mordant in the textile industry, pharmaceutical emetic agents, astringents, fungicides and wood and leather preservatives. It can be derived from the reaction between zinc or zinc oxide and sulfuric acid or from the baking of sphalerite in the baking furnace followed by extraction and refining.
Uses
Zinc sulfate occurs in nature as the mineral, zinkosite. The heptahydrate, ZnSO4•7H2O is the mineral, goslarite. The salt is used as a mordant in calico-printing, in making rayon, in preserving wood, in animal feeds, in electroplating, and in preparing many zinc compounds.
Indications
- Convergence preservatives: as eye drops, can be used for the treatment of conjunctivitis, trachoma, nasal blepharitis and so on.
- Oral stimulation of gastric mucosa can cause reflex vomiting. It can be used as emetic drug, now less used.
- It can be used for the treatment of zinc deficiency: zinc is the ingredient of many important enzymes in vivo such as carbonic anhydrase and alkaline phosphatase, being an indispensable trace element in the human body. Supplementation of zinc can be used for treating zinc deficiency such as dwarfism, acral dermatitis and zinc deficiency caused by long-term vein nutritional deficiency and so on.
- It can be used for the treatment of zinc deficiency related diseases: such as acne vulgaris, skin ulcers (venous, arterial, leprosy), psoriasis, seborrheic dermatitis, chronic eczema, oral ulcers, hair loss and smell taste disorders.
- It can be used as mordant for printing and dyeing, wood preservative, bleaching agent for papermaking, also used in medicine, artificial fiber, electrolysis, electroplating, and pesticide as well as zinc salt production.
Production Methods
Zinc sulfate is produced as an intermediate in recovering zinc from mineral zinc blende, ZnS (see Zinc, Recovery). The mineral is roasted at about 1,000°C to form zinc oxide and sulfur dioxide which, on prolonged heating in excess air, converts to zinc sulfate:
2ZnS + 3O2 → 2ZnO + 2SO2
992 ZINC SULFATE2ZnO + 2SO2 + O2 → 2ZnSO4
In the zinc recovery process, roasted products are leached with sulfuric acid, whereupon zinc oxide is converted to sulfate.
ZnO + H2SO4 → ZnSO4 + H2O
Also, zinc sulfate can be prepared by reacting metallic zinc with dilute sulfuric acid followed by evaporation and crystallization:
Zn + H2SO4 → ZnSO4 + H2
Chemical Properties
used in zinc plating and as a mordant [KIR84]
Physical properties
The anhydrous sulfate is a colorless rhombohedral crystalline solid; refractive index 1.658; density 3.54 g/cm3; decomposes at 600°C; soluble in water, methanol, and glycerol.
The heptahydrate, ZnSO4?7H2O, is a colorless crystalline solid; metallic taste; rhombohedral crystals; effloresces; refractive index 1.457; density 1.957 g/cm3 at 25°C; melts at 100°C; loses all its water molecules at 280°C; decomposes above 500°C; very soluble in water, 96.5 g/100mL at 20°C; soluble in glycerol, 40 g/100 mL; insoluble in alcohol
The hexahydrate, ZnSO4?6H2O constitutes colorless monoclinic or tetragonal crystals; density 2.072 g/cm3at 15°C; loses five water molecules at 70°C; soluble in water.
Uses
The principal commercial preparation of zinc sulfate is the monohydrate granular (36% of zinc) used as a fertilizer. It is also a component of spinning bath in the production of rayon, carbamate fungicides, zinc plating baths, and ophthalmic solutions. Zinc sulfate is used as an accelerating agent in dental impression material, froth flotation agent, and animal feed additive.
Uses
Zinc sulfate solution has been used as a component in media for in vitro hyphal growth assays. It has also been used as a supplement in the media along with isopropyl β-D-1-thiogalactopyranoside (IPTG), while expressing zinc containing proteins.
Uses
Used in conjunction with barium hydroxide to deproteinize via barium sulfate precipitation whole blood, plasma, or serum samples which are colored or turbid. The resulting supernatant or filtrate is then available for subsequent analysis of glucose by Somogi-Nelson method.
Definition
zinc sulphate: A white crystalline water-soluble compound made by heating zinc sulphide ore in air and dissolving out and recrystallizing the sulphate. The common form is the heptahydrate, ZnSO4.7H2O; r.d. 1.9. This loses water above 30°C to give the hexahydrate and more water is lost above 70°C to form the monohydrate. The anhydrous salt forms at 280°C and this decomposes above 500°C. The compound, which was formerly called white vitriol, is used as a mordant and as a styptic (to check bleeding).
Production Methods
Zinc sulfate is produced from side streams of electrolytic zinc manufacture. The main source of secondary zinc for zinc sulfate production is galvanizer residues. ZnSO4 is available as the mono-, hexa- and heptahydrates with zinc contents of 36%, 24%, and 22%, respectively.
Definition
ChEBI: A metal sulfate compound having zinc(2+) as the counterion.
General Description
Anhydrous Zinc sulphate is a colorless crystalline solid. Zinc sulphate is also obtained as a hexahydrate, ZnSO4.6H2O, and as a heptahydrate ZnSO4.7H2O. All forms are soluble in water. All are noncombustible. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Zinc sulphate is used in the production of rayon, as a feed supplement, used to obtaine lectrolyte zinc, in printing textiles and to make lithopone, to impregnate wood and hides,as an additive to spinning baths for production of synthetic silks, in electroplating, and in animal feeds.
Air & Water Reactions
Water soluble. Efflorescent in air. Aqueous solutions are acidic.
Reactivity Profile
Acidic salts, such as Zinc sulphate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Inhalation of dust causes irritation of nose and throat. Ingestion can cause irritation or corrosion of the alimentary tract. Contact with eyes or skin causes irritation.
reaction suitability
reaction type: Redox Reactions
Agricultural Uses
Zinc sulphate is a white, crystalline, water-soluble
compound made by heating zinc sulphide ore
in air and dissolving the sulphate formed, and
crystallizing it.
Zinc sulphate is the most common zinc salt (about
35% of zinc) used for preventing zinc deficiency in
plants. It is sprayed on the foliage as a water solution or
added in large quantities directly to the soil.
Agricultural Uses
White vitriol is another name for zinc sulphate heptahydrate, and is a commonly used zinc salt. It is widely used as a fertilizer for overcoming zinc deficiency.
Industrial uses
Ferro sulfate (FeSO4·7H2O) is a crystalline substance greenish in color, with a specific gravity of 1.899. Ferro sulfate is obtained from various solutions using a vacuum crystallization method. Ferro sulfate has been used as a depressant and co-depressant in the following applications: (a) depression of sphalerite together with cyanide , (b) depression of fine molybdenite also with cyanide, and (c) in copper/lead separation using a method, based on copper depression by cyanide.
Biochem/physiol Actions
Zinc sulfate?solution is as potent as formalin. This chemical is mainly used to treat footrot. It may also be used to treat acute bronchiolitis.
Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Human systemic effects by ingestion: acute pulmonary edema, agranulocytosis, blood pressure decrease, diarrhea and other gastrointestinal changes, hypermotility, increased pulse rate without blood pressure decrease, level changes for metals other than Na/K/Fe/Ca/P/Cl, microcytosis with or without anemia, normocytic anemia. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. An eye irritant. When heated to decomposition it emits toxic fumes of SOx and ZnO. See also SULFATES and ZINC COMPOUNDS.
Purification Methods
Crystallise it from aqueous EtOH or dilute H2SO4 below 39o when it forms the heptahydrate, and between 39o and 70o it forms the hexahydrate, and above 70o the monohydrate is stable. The anhydrous salt is obtained from the hydrates by heating at 280o or lower temperatures in a current of dry air. It decomposes to ZnO and SO2 at 767o. The solubility of the heptahydrate in H2O is 5.88% at 0o, 61.92% at 30o, 66.61% at 35o and 70.05% at 39o.
Zinc sulphate Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12809 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5868 | 58 |
| Chongqing Chuandong Chemical (Group) Co. Ltd. | +86-13637972665 +86-13637972665 | wxc@cd1958.com | China | 81 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-17396673057 | linda@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
| Hebei Andu Technology Com.,Ltd | +86-17798073498 +86-17798073498 | admin@hbandu.com | China | 378 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21628 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 | sales@amoychem.com | China | 6383 | 58 |
Related articles
- Zinc sulfate: Exploration of the mysteries of multifunctional inorganic compounds
- This paper aims to comprehensively review the properties, preparation methods, application fields, and future development tren....
- Jul 2,2024
View Lastest Price from Zinc sulphate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-24 | Zinc sulphate
7733-02-0
|
US $3.50 / kg | 1kg | ≥98% | 3000tons/month | Hebei Andu Technology Com.,Ltd | |
 |
2025-04-23 | ZINC SULPHATE
7733-02-0
|
US $1.00 / kg | 1kg | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-15 | Zinc sulphate
7733-02-0
|
US $70.00 / kg | 1kg | 99 | 5000 | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- Zinc sulphate
7733-02-0
- US $3.50 / kg
- ≥98%
- Hebei Andu Technology Com.,Ltd
-

- ZINC SULPHATE
7733-02-0
- US $1.00 / kg
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Zinc sulphate
7733-02-0
- US $70.00 / kg
- 99
- Hebei Zhuanglai Chemical Trading Co.,Ltd