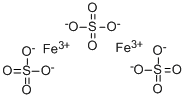Ferric sulfate
- CAS No.
- 10028-22-5
- Chemical Name:
- Ferric sulfate
- Synonyms
- Fe2(SO4)3;FERRIC SULPHATE;liusuantie;IRON (III) SULFATE;diiron tris(sulphate);Polymerized ferrous sulfate;NA-9121;FERIX-3;BETA FLOC;MONSEL SALT
- CBNumber:
- CB3496798
- Molecular Formula:
- Fe2O12S3
- Molecular Weight:
- 399.88
- MDL Number:
- MFCD00011007
- MOL File:
- 10028-22-5.mol
- MSDS File:
- SDS
| Melting point | 480°C |
|---|---|
| Boiling point | 101-118 °C |
| Density | 3.097 |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | slightly soluble in ethanol; insoluble in acetone |
| form | Powder |
| color | Yellow-gray |
| Water Solubility | Soluble in water. Sparingly soluble in alcohol. Almost insoluble in acetone and ethyl acetate. Insoluble in sulfuric acid and ammonia. |
| Sensitive | Hygroscopic |
| Merck | 14,4032 |
| Exposure limits |
ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| LogP | -1.031 (est) |
| CAS DataBase Reference | 10028-22-5(CAS DataBase Reference) |
| FDA 21 CFR | 184.1307 |
| Substances Added to Food (formerly EAFUS) | FERRIC SULFATE |
| SCOGS (Select Committee on GRAS Substances) | Ferric sulfate (packaging) |
| EWG's Food Scores | 1 |
| FDA UNII | 4YKQ1X5E5Y |
| NIST Chemistry Reference | Ferric sulfate(10028-22-5) |
| EPA Substance Registry System | Ferric sulfate (10028-22-5) |
| Pesticides Freedom of Information Act (FOIA) | Ferric sulfate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37 | |||||||||
| Safety Statements | 26 | |||||||||
| RIDADR | UN 9121 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NO8520000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2833298080 | |||||||||
| Hazardous Substances Data | 10028-22-5(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Ferric sulfate price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | 010717 | Iron(III) sulfate, Puratronic?, 99.998% (metals basis) | 10028-22-5 | 5g | $96.2 | 2021-12-16 | Buy |
| Alfa Aesar | 010717 | Iron(III) sulfate, Puratronic?, 99.998% (metals basis) | 10028-22-5 | 25g | $306 | 2021-12-16 | Buy |
| GFS CHEMICALS | 39502 | FERRICSULFATE,HYD.,REAG | 10028-22-5 | 2.5kg | $384.69 | 2021-12-16 | Buy |
| GFS CHEMICALS | 39501 | FERRICSULFATE,HYD.,REAG | 10028-22-5 | 500g | $108 | 2021-12-16 | Buy |
| GFS CHEMICALS | 39505 | FERRICSULFATE,HYD.,REAG | 10028-22-5 | 100g | $42.19 | 2021-12-16 | Buy |
Ferric sulfate Chemical Properties,Uses,Production
Description
Ferric sulfate (iron sulfate) is the ferric salt of sulfate. It can be used as a mordant in dyeing and used as a coagulant for the treatment of industrial wastes. In the medical field, it can be used as an astringent and styptic. It is a kind of hemostatic agent being capable of controlling or stopping the flow of blood. It is also recommended that ferric sulfate is a good pulpotomy agent in primary teeth with good potential of substituting the formocresol. It can also be used in pigment, and in pickling baths for aluminum and steel. It is generally prepared through the reaction between ferrous sulfate, sulfuric acid and an oxidizing agent such as nitric acid.
References
Zouboulis, A. I., P. A. Moussas, and F. Vasilakou. "Polyferric sulphate: preparation, characterisation and application in coagulation experiments."Journal of Hazardous Materials 155.3 (2008):459. Zhang, Nianrong, D. Du, and Q. Wang. "Mordant Dyeing Behavior of Tanning Extracts on Leather Dyeing." China Leather (2012). Rodríguez-Priego, M. E., et al. "Ferric sulphate alterations on primary dentin and the adhesive interface. " Journal of Adhesive Dentistry16.4(2014):347-356.
Dan, E. Fischer. "Ferric sulfate as hemostatic agent." US, US 4551100 A. 1985.
Lee, Sang Heon, M. N. Lee, and S. H. Lee. "PRIMANY TOOTH PULPOTOMY USING FERRIC SULFATE." Journal of the Korean Academy of Pedtatric Dentistry 25.4(1998):843-848.
Description
§184.1307(a) Ferric sulfate (iron(HI)sulfate, Fe2(S04)3), a yellow substance that may be prepared by oxidizing iron(II)sulfate or by treating ferric oxide or ferric hydroxide with sulfuric acid.
Chemical Properties
Ferric Sulfate is a grayish-white powder or yellow lumpy crystals.
Chemical Properties
Yellow crystals or grayish-white powder.(1) slightly soluble in water, (2) very soluble in water. Keep well closed and protected from light. Noncombustible.
Physical properties
The anhydrous salt constitutes grayish-white rhombic crystals; hygroscopic; density 3.10 g/cm3; slightly soluble in cold water; decomposes in hot water. The nonahydrate is a yellow hexagonal crystalline substance; refractive index 1.54; density 2.10 g/cm3; hardness 2.5 Mohs; decomposes at 400°C; very soluble in water.
Uses
Iron(III) Sulfate can be used for water treatment system.
Uses
Ferric Sulfate is a nutrient and dietary supplement that is a source of iron.
Uses
In preparation of iron alums, other iron salts and pigments; as coagulant in water purification and sewage treatment; in etching aluminum; in pickling stainless steel and copper; as mordant in textile dyeing and calico printing; in soil conditioners; as polymerization catalyst.
Definition
Ferrous sulfate,also known as ferrisulpas, green copperas, green vitriol, iron sulfate, and melanterite, is composed of blue green monoclinic crystals. It is soluble in water and is used as a mordant for dyeing wool in the textile industry. Ferrous sulfate is also used as a disinfectant and in the manufacture of ink.
Preparation
Iron(III) sulfate may be prepared by oxidation of iron(II) sulfate by hydrogen peroxide, nitric acid or any other suitable oxidizing agent. The reaction is carried out in sulfuric acid. Balanced molecular equations for the reactions with hydrogen peroxide and nitric acid are as follows:
2FeSO4 + H2SO4 + H2O2 → Fe2(SO4)3 + 2H2O
6FeSO4 + 3H2SO4 + 2HNO3 → 3Fe2(SO4)3 + 2NO + 4H2O
Even in the absence of an oxidizing agent, concentrated sulfuric acid alone can1 convert iron(II) sulfate to iron(III) sulfate: 2FeSO4 + 2H2SO4→ Fe2(SO4)3 + SO2 + 2H2O It also may be prepared by treating iron(III) oxide with sulfuric acid:
Fe2O3 + 3H2SO4 → Fe2(SO4)3 + 3H2O
.
General Description
A yellow crystalline solid or a grayish-white powder. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ferric sulfate is used for water purification, and as a soil conditioner.
Air & Water Reactions
Soluble in water. Hygroscopic in air. Forms acidic aqueous solutions.
Reactivity Profile
Ferric sulfate is acidic. Corrosive to copper, copper alloys, mild steel, and galvanized steel [USCG, 1999].
Health Hazard
Inhalation of dust irritates nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes and can irritate skin on prolonged contact.
Flammability and Explosibility
Non flammable
Agricultural Uses
Herbicide, Molluscicide, Agricultural product constituent: Ferric sulfate is used on forage alfalfa, almonds, nurseries and structural pest control. This material is also used in pigments, textile dyeing, water treatment, and metal pickling
Trade name
GREENMASTER AUTUMN®; MAXICROP MOSS KILLER®; VITAX MICRO GRAN®; VITAX TURF TONIC®
Safety Profile
A poison by intraperitoneal route. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx and Fe-. See also SULFATES and other ferric salts.
Potential Exposure
This material is used in pigments, textile dyeing, water treatment; and metal pickling.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Thesymptoms of metal fume fever may be delayed for 4-12 hfollowing exposure: it may last less than 36 h.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoning from iron.
storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from light,moisture, aluminum, magnesium, copper and its alloys,zinc, galvanized and mild steels.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz ardous material, Technical Name Required.
Purification Methods
Dissolve the sulfate in the minimum volume of dilute aqueousH2SO4 and allow it to evaporate at room temperature until yellowish-white crystals start to form. Do not concentrate by boiling off the H2O as basic salts will be formed. Various hydrates are formed; the common ones are the dodeca and nona hydrates which are violet in colour. The anhydrous salt is colourless and is very hygroscopic, but it dissolves in H2O slowly unless ferrous sulfate is added. [Gmelin’s, Iron (8th edn) pp 439-462 1932.]
Incompatibilities
Hydrolyzed slowly in aqueous solution. Incompatible with magnesium, aluminum. Corrosive to copper and its alloys, mild and galvanized steel. Light sensitive.
Waste Disposal
Treat with soda ash or dilute NaOH. Separate any precipitate and landfill. Flush solution to sewer.
Ferric sulfate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 | allison@yan-xi.com | China | 5854 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18137 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2097 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | niki@zlchemi.com | China | 7245 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2957 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 | sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
View Lastest Price from Ferric sulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-16 | Ferric sulfate
10028-22-5
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-16 | Ferric sulfate
10028-22-5
|
US $0.00 / Kg/Bag | 1KG | 99% | 10000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2025-04-16 | Ferric sulfate
10028-22-5
|
US $45.00 / kg | 1kg | 99% | 20 tons | Hebei Zhuanglai Chemical Trading Co Ltd |
-

- Ferric sulfate
10028-22-5
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Ferric sulfate
10028-22-5
- US $0.00 / Kg/Bag
- 99%
- Jinan Finer Chemical Co., Ltd
-

- Ferric sulfate
10028-22-5
- US $45.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co Ltd