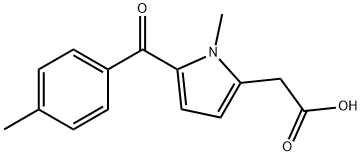
Tolmetin
- CAS No.
- 26171-23-3
- Chemical Name:
- Tolmetin
- Synonyms
- C07149;olmetin;McN 2559;Tolmetin;Methyiin;Tolmetine;TOLMETINUM;pyrolyzate;Tolmetin, 10 mM in DMSO;Tolmetin Solution, 100ppm
- CBNumber:
- CB2875183
- Molecular Formula:
- C15H15NO3
- Molecular Weight:
- 257.28
- MDL Number:
- MFCD00599595
- MOL File:
- 26171-23-3.mol
| Melting point | 156 °C |
|---|---|
| Boiling point | 400.53°C (rough estimate) |
| Density | 1.1391 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 3.5(H2O t undefined I undefined) (Uncertain) |
| color | Off-White to Pale Yellow |
| Water Solubility | 222 mg/L |
| FDA UNII | D8K2JPN18B |
| ATC code | M01AB03,M02AA21 |
| NIST Chemistry Reference | Tolmetin(26171-23-3) |
| EPA Substance Registry System | 1H-Pyrrole-2-acetic acid, 1-methyl-5-(4-methylbenzoyl)- (26171-23-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H302-H335-H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362-P264-P270-P301+P312-P330-P501 |
| Hazardous Substances Data | 26171-23-3(Hazardous Substances Data) |
Tolmetin price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | M332943 | 1-Methyl-5-p-toluoylpyrrole-2-aceticAcid | 26171-23-3 | 100mg | $70 | 2021-12-16 | Buy |
| AK Scientific | J10499 | Tolmetin | 26171-23-3 | 5mg | $39 | 2021-12-16 | Buy |
| Matrix Scientific | 074159 | 1-Methyl-5-p-toluoylpyrrole-2-acetic acid 95+% | 26171-23-3 | 1g | $278 | 2021-12-16 | Buy |
| ChemScene | CS-W008734 | Tolmetin ≥98.0% | 26171-23-3 | 25g | $504 | 2021-12-16 | Buy |
| Matrix Scientific | 074159 | 1-Methyl-5-p-toluoylpyrrole-2-acetic acid 95+% | 26171-23-3 | 10g | $1000 | 2021-12-16 | Buy |
Tolmetin Chemical Properties,Uses,Production
Description
An antiinflammatory, analgesic, and antipyretic that is as efficacious as moderate doses of aspirin and better tolerated. Tolmetin produces a number of adverse effects including epigastric pain, dyspepsia, nausea, and vomiting. Tolmetin is approximately 99% plasma protein bound, yet does not interfere with concurrent treatment with oral hypoglycemics. Tolmetin has been found to be effective in the treatment of osteoarthritis and rheumatoid arthritis.
Originator
Tolectin,McNeil,US,1976
Uses
inhibits synthesis of prostaglandins and exhibits expressed analgesic, anti-inflammatory, and fever-reducing properties. It is used for relieving weak to moderate pain in rheumatoid arthritis and osteoarthritis.
Uses
Tolmetine is an NSAID.
Indications
Tolmetin (Tolectin) is indicated for the relief of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, and moderate pain. It is ineffective in gouty arthritis for unknown reasons.Tolmetin can inhibit both COX-1 and COX-2 but has a moderate selectivity for COX-1. The most frequently reported side effects are GI disturbance and CNS reactions (e.g., headache, asthenia, and dizziness). These effects are less frequently observed than after aspirin or indomethacin use. Blood pressure elevation, edema, and weight gain or loss have been associated with tolmetin administration. Tolmetin metabolites in urine have been found to produce pseudoproteinuria in some laboratory tests.
Definition
ChEBI: A monocarboxylic acid that is (1-methylpyrrol-2-yl)acetic acid substituted at position 5 on the pyrrole ring by a 4-methylbenzoyl group. Used in the form of its sodium salt dihydrate as a nonselective nonsteroidal anti-inflammatory drug.
Manufacturing Process
5-(p-Toluoyl)-1-methylpyrrole-2-acetonitrile - To a cooled suspension of 26.6 g
(0.2 mol) aluminum chloride in 80 ml dichloroethane is added dropwise 30.8 g
(0.2 mol) p-toluoyl chloride. The resulting solution is added dropwise to a
solution of 1-methylpyrrole-2-acetonitrile in 80 ml dichloroethane cooled
externally with an ice bath. After the addition, the resulting solution is stirred
at room temperature for 20 minutes and then refluxed for 3 minutes. The
solution is poured into ice acidified with dilute hydrochloric acid. The organic
and aqueous fractions are separated. The aqueous fraction is extracted once
with chloroform.
The organic fractions are combined and washed successively with N,N_x0002_dimethyl-1,3-propanediamine, dilute hydrochloric acid, saturated sodium
bicarbonate solution and saturated sodium chloride solution. The organic
fraction is dried over anhydrous magnesium sulfate. The solvent is then
evaporated off. Upon trituration of the residue with methanol, a solid
crystallizes, 5-(p-toluoyl)-1-methylpyrrole-2-acetonitrile, which is removed by
filtration and purified by recrystallization from benzene.
Additional product is isolated from the mother liquors which are combined,
concentrated in vacuo and the resulting oily residue column chromatographed
on neutral alumina using hexane, benzene and ether as successive solvents.
The product is isolated by concentrating in vacuo the first few major
compound-bearing fractions (10% ether in benzene). The solids are combined
and recrystallized from methanol and then from benzene-hexane, melting
point 102°C to 105°C.
5-(p-Toluoyl)-1-methylpyrrole-2-acetic acid - A solution of 3.67 g (0.015 mol)
of 5-(p-toluoyl)-1-methylpyrrole-2-acetonitrile, 24 ml of 1 N sodium hydroxide
and 50 ml of 95% ethanol is stirred and refluxed for 24 hours.
The resulting solution is poured into ice acidified with dilute hydrochloric acid.
A white solid precipitates which is extracted into ether. The ether phase is
washed with a saturated solution of sodium chloride and dried over anhydrous
magnesium sulfate. The solvent is evaporated and a white solid, 5-(p-toluoyl)-
1-methylpyrrole-2-acetic acid is obtained which is recrystallized twice from
isopropanol, melting point 155°C to 157°C.
Therapeutic Function
Antiinflammatory
Biological Functions
Tolmetin (Tolectin) is an antiinflammatory, analgesic, and antipyretic agent that produces the usual gastric distress and ulceration observed with NSAIDs. However, tolmetin is better tolerated than aspirin and produces less tinnitus and vertigo. Tolmetin is a substitute for indomethacin in indomethacin-sensitive patients and is unique among such drugs in that it can be used to treat juvenile arthritis.
General Description
Tolmetin sodium (Tolectin), is an arylacetic acid derivativewith a pyrrole as the aryl group. This drug is well absorbed and has a relatively short plasma half-life (1 hour). It is recommendedfor use in the management of acute and chronicRA. Its efficacy is similar to aspirin and indomethacin, butwith less frequency of the adverse effects and tinnitus associatedwith aspirin. It does not potentiate coumarin-likedrugs nor alter the blood levels of sulfonylureas or insulin.However, tolmetin, and especially its closely related drug,zomepirac (i.e., with a p-chlorobenzoyl group and an additionalmethyl group on the pyrrole ring), can produce a rarebut fatal anaphylactic reaction because of irreversible bindingof their unstable acyl glucuronides. Zomepirac waswithdrawn from market because it is eliminated only via theester-type, acyl glucuronide. It is possible that tolmetin isless toxic in this regard because it undergoes additional hepaticbenzylic hydroxylation via its p-methyl group and isexcreted as its stable ether glucuronide.
Trade name
Artrocaptin (Estedi, Spain), Tolectin (Cilag, Belgium; Janssen-Cilag, Austria; McNeil, USA).
Mechanism of action
Tolmetin inhibits both isoforms of cyclooxygenase with some preference for COX-1 .
Pharmacology
Tolmetin is administered orally, rectally, or topically (600–1800 mg/d). The peak plasma concentrations are reached within 30 to 60 min. Tolmetin shows a high plasma protein binding of 99% and a biphasic plasma half-life of 1 to 2 and 5h, respectively.
Clinical Use
Tolmetin is a nonsteroidal anti-inflammatory drug used for the treatment of mild to moderate pain states in musculoskeletal, soft-tissue, and joint disorders like rheumatoid arthritis, osteoarthritis, and gout as well as juvenile rheumatoid arthritis.
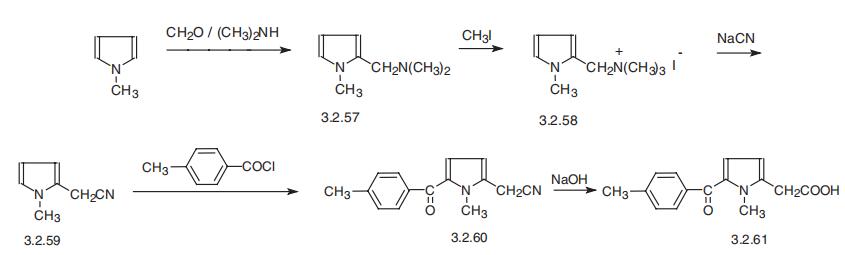
Synthesis
Tolmetin, 1-methyl-5-n-tolylpyrrol-2-acetic acid (3.2.61) is synthesized from 1- methylindole, which is aminomethylated using formaldehyde and dimethylamine, forming 2-dimethylaminomethyl-1-methylindol (3.2.57). The product is methylated by methyl iodide, giving the corresponding quaternary salt (3.2.58). Reaction of the product with sodium cyanide gives 1-methylpyrrole-2-acetonitrile (3.2.59), which is acylated at the free |á-position of the pyrrole ring by 4-methylbenzoylchloride in the presence of aluminum chloride. The resulting 1-methyl-5-n-toluylpyrrol-2-acetonitrile (3.2.60) undergoes further alkaline hydrolysis, giving corresponding acid, tolmetin (3.2.61) [116¨C118].
Tolmetin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12816 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Taizhou Tianhong Biochemistry Technology Co., Ltd. | 0523-86132544 | sales@thbiochem.com | CHINA | 305 | 60 |
| NINGBO INNO PHARMCHEM CO., LTD. | 13867897135 | sales@nbinno.com | CHINA | 923 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29808 | 58 |
View Lastest Price from Tolmetin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-02 | Tolmetin
26171-23-3
|
US $10.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2025-03-31 | Tolmetin
26171-23-3
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-03-21 | Tolmetin
26171-23-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd |