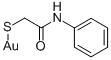
Aurothioglycanide
- CAS No.
- 16925-51-2
- Chemical Name:
- Aurothioglycanide
- Synonyms
- Aurothioglycanide;[[(Phenylcarbamoyl)methyl]thio]gold(I)
- CBNumber:
- CB31178062
- Molecular Formula:
- Molecular Weight:
- 0
- MDL Number:
- MFCD00864770
- MOL File:
- 16925-51-2.mol
| Melting point | 238-241° |
|---|---|
| FDA UNII | 2A9D34A16M |
Aurothioglycanide Chemical Properties,Uses,Production
Originator
Lauron,Endo,US,1945
Manufacturing Process
The product is made preferably by reacting thioglycolic acid anilide with an
aurous bromide (AuBr).
Prior art methods for making the starting material, HSCH2CONHC6H5 are
disclosed in an article by Beckurts et al. in Journ. Praktische Chemie (2) 66 p.
174, and in the literature referred to in the mentioned article.
Ten grams of the potassium salt of bromoauric acid are dissolved in 100 cc of
96% ethyl alcohol. This salt is also designated as potassium auribromide.
Sulfur dioxide (SO2) is then led through this solution, through a fine capillary
tube, for several minutes. This reaction produces aurous bromide (AuBr). The
solution of the aurous bromide is then allowed to stand for 2 to 3 hours until
it is colorless. A precipitate of KBr is thus formed. This precipitate is separated
from the solution of the aurous bromide which is added to a solution of three
grams of the thioglycolic acid anilide in 50 cc of ethyl alcohol. This is done at
about 20°C. Then 300 cc of water are added to this mixture, at 20°C. The
water is then removed by decantation or any suitable method, and the
mixture is repeatedly thus treated with water, in order to remove all impurities
which can thus be removed. The product is then centrifuged twice with 96%
ethyl alcohol. It is then centrifuged three times with 100% or absolute ethyl
alcohol, and then centrifuged three times with water-free ligroin (petroleum
ether), i.e., the 40-60°C fraction which is distilled from petroleum. After each
centrifuging, the product is separated from the liquid which has been used
during the centrifuging.
The product is then dried in a high vacuum with the use of phosphorus
pentoxide (P2O5).
Therapeutic Function
Antiarthritic
Aurothioglycanide Preparation Products And Raw materials
Aurothioglycanide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Lanospharma Laboratories Co.,Ltd | -- | sales@lanospharma.com | China | 6343 | 56 |
| Shanghai New Union Textra Import & Export Co., Ltd | -- | zhou@pharmchemical.com | China | 2752 | 60 |
| Supplier | Advantage |
|---|---|
| Lanospharma Laboratories Co.,Ltd | 56 |
| Shanghai New Union Textra Import & Export Co., Ltd | 60 |