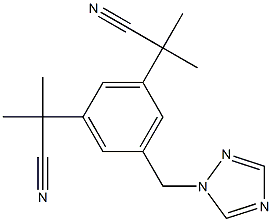
Anastrozole
- CAS No.
- 120511-73-1
- Chemical Name:
- Anastrozole
- Synonyms
- ARIMIDEX;ANASTRAZOLE;Anastrozol;MDPT;zd;Anatrozole;Arimidex(Anastrozole);ZD-1033;Anastrol;Astrozole
- CBNumber:
- CB3121715
- Molecular Formula:
- C17H19N5
- Molecular Weight:
- 293.37
- MDL Number:
- MFCD00866298
- MOL File:
- 120511-73-1.mol
- MSDS File:
- SDS
| Melting point | 81-82°C |
|---|---|
| Boiling point | 469.7±55.0 °C(Predicted) |
| Density | 1.08±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: soluble40mg/mL |
| pka | 2.62±0.10(Predicted) |
| form | solid |
| color | White to off-white |
| BCS Class | 1 (LogP), 3 (CLogP) |
| InChI | InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3 |
| InChIKey | YBBLVLTVTVSKRW-UHFFFAOYSA-N |
| SMILES | C(#N)C(C1=CC(CN2C=NC=N2)=CC(C(C#N)(C)C)=C1)(C)C |
| CAS DataBase Reference | 120511-73-1(CAS DataBase Reference) |
| FDA UNII | 2Z07MYW1AZ |
| NCI Dictionary of Cancer Terms | anastrozole; Arimidex |
| NCI Drug Dictionary | anastrozole |
| ATC code | L02BG03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H360FD | |||||||||
| Precautionary statements | P202-P264-P270-P280-P301+P312-P308+P313 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-22-61-60 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CZ1465000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29339980 | |||||||||
| NFPA 704 |
|
Anastrozole price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A2736 | Anastrozole ≥98% (HPLC) | 120511-73-1 | 10mg | $106 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1034807 | Anastrozole | 120511-73-1 | 200mg | $706 | 2024-03-01 | Buy |
| Cayman Chemical | 11987 | Anastrozole ≥98% | 120511-73-1 | 10mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 11987 | Anastrozole ≥98% | 120511-73-1 | 50mg | $121 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0001522 | Anastrozole European Pharmacopoeia (EP) Reference Standard | 120511-73-1 | y0001522 | $153 | 2024-03-01 | Buy |
Anastrozole Chemical Properties,Uses,Production
Description
Anastrozole (120511-73-1), chemically known as 2,2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropiononitrile) a derivative of benzotriazole with the CAS registry number 120511-73-1, is a non-steroidal, third-generation achiral triazole derivative marketed as ARIMIDEX? by AstraZeneca Pharmaceuticals LP1. It is one of the third-generation aromatase inhibitor which is a highly competitive and selective inhibitor of aromatase, thus blocking the conversion of testosterone into estradiol and androstenedione into estrone. Inhibition of the aromatase enzyme occurs particularly through competitive binding of aromatase to the hemegroup of cytochrome P450, decreasing estrogen biosynthesis in the peripheral tissues of the body and in the breast.
Treatment for Early-stage Breast Cancer
Anastrozole is a generally well tolerated treatment for early-stage breast cancer. Like other aromatase inhibitors, its most important adverse effect was an increased risk of bone fractures, which for anastrozole was restricted to the treatment period. It characteristically has mild toxicity when compared with chemotherapy; however, it have been noticed that more patients treated with anastrozole have complained of joint symptoms than expected, particularly digital stiffness similar to that of rheumatoid arthritis. Some clinical trials of anastrozole for postmenopausal women with breast cancer in Europe and the United states reported musculoskeletal disorders as adverse events.
Anastrozole (Arimidex®) is an aromatase inhibitor approved in the EU, the US and in other countries worldwide for use as an adjuvant treatment in postmenopausal women with early-stage, hormone receptor-positive breast cancer. It is also approved in the EU and other countries worldwide for continuing adjuvant treatment in women who have already had 2–3 years of adjuvant tamoxifen treatment for breast cancer.
Uses
Anastrozole has significant effects on breast cancer treatment and, therefore, it is currently used as first-line treatment in estrogen receptor (ER)-positive postmenopausal women, particularly to treat locally advanced or metastatic breast cancer. Furthermore, it is also indicated for early cancer treatment, tumor chemoprevention and postmenopausal women using tamoxifen, especially if the drug is used during a prolonged period of time and has been indicated in the disease’s recurrence, i.e., as another therapeutic endocrine option.
Pharmacokinetics
Anastrozole has linear pharmacokinetics. It is metabolized primarily in the liver, with a plasma elimination half-life of 40–50 hours, indicating that oncedaily administration is adequate. In vitro and clinical studies indicate that drugdrug interactions are unlikely to occur between anastrozole and drugs metabolized by hepatic cytochrome P450 enzymes. In patients with breast cancer, there were no clinically important interactions between anastrozole and tamoxifen or its metabolite, N-desmethyltamoxifen.
Indications and Usage
Anastrozole(120511-73-1) is a potent selective triazole aromatase inhibitor that inhibits the aromatase that cytochrome P-450 is dependent on to prevent the biosynthesis of estrogen. Estrogen is the main stimulating factor for breast cancer cell growth. This drug’s half maximal inhibitory concentration (IC50) value to human placental aromatase is 15nmol/L. Compared to the traditional drug tamoxifen, Anastrozole can comprehensively and effectively lower the risks of breast cancer recurrence and metastasis, thus extending patients’ disease-free survival.
Anastrozole is suitable for treating postmenopausal advanced breast cancer patients, especially postmenopausal advanced breast cancer patients who experienced recurrence following hormone assisted therapy.
Clinical Research
A series of clinical trials compared the effects of third generation aromatase inhibitor Anastrozole with those of tamoxifen. The trial was a global multicenter trial and includes 381 centers in 21 countries. Since 1996, there have been 9366 patients who have participated, and the follow-up time is 100 months. 25.8% of patients in the Anastrozole group experienced recurrence, while 29.9% of patients in the tamoxifen group did. Thus, Anastrozole’s recurrence rate was 4.1% lower than that of tamoxifen, and its risk of distant metastasis was lower as well. Anastrozole is also safer than tamoxifen.
Description
Anastrozole(120511-73-1) was first approved for use in the United States in 1995 for the treatment of advanced breast cancer in post-menopausal women. Anastrozole is a highly potent and selective aromatase inhibitor. It is extremely potent in lowering circulating estradiol to undetectable levels in treated patients without altering other circulating hormones. The drug is reportedly well absorbed and tolerated following oral administration.
Chemical Properties
Crystalline Solid. soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. The solubility of anastrozole in these solvents is approximately 20, 13, and 14 mg/ml.
Originator
Zeneca (United Kingdom)
Uses
Anastrozole is a nonsteroidal inhibitor of aromatase which effectively blocks estrogen synthesis in postmenopausal women and is used as therapy of estrogen receptor positive breast cancer. Anastrozole has been associated with a low rate of serum enzyme elevations during therapy and rare instances of clinically apparent liver injury.
Definition
ChEBI: Anastrozole is a 1,2,4-triazole compound having a 3,5-bis(2-cyano-2-propyl)benzyl group at the 1-position. It has a role as an antineoplastic agent and an EC 1.14.14.14 (aromatase) inhibitor. It is a member of triazoles and a nitrile.
Preparation
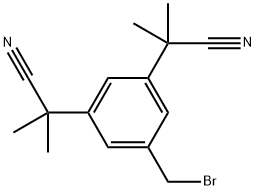
synthesis of anastrozole can be realized in four steps based on 3,5-bis(bromomethyl)toluene. Starting with a S N 2 displacement using potassium nitrile and tetrabutylammonium bromide as a phase transfer catalyst to give bis-nitrile compound. Bis-nitrile compound formed undergoes deprotonation with NaH and methylated afterwards with methyl iodide to give bis-dimethyated product.Product undergoes radical substitution reaction following the Wohl-Ziegler reaction using N-bromosuccinamide and benzoyl peroxide as the radical initiator.In the final step, benzylbromide undergoes SN2 displacement with sodium triazole to give anastrozole.
Application
Anastrozole (aromatase inhibitor) has been used:
as a positive control in DNA fragmentation (ladder) assay
to investigate its effects along with extra virgin olive oil and its major fatty acid component (omega-9 OA) in estrogen receptor positive mammary adenocarcinoma cells
to study its effects on viability, cell proliferation and apoptosis in Glioblastoma multiforme model in vivo
brand name
Arimidex (AstraZeneca).
Therapeutic Function
Antitumor
General Description
Anastrozole(120511-73-1) is a non-steroidal and expensive drug marketed under the trade name Arimidex. It was the first specific aromatase inhibitor approved in theUnited States. It is indicated for first-line treatment of postmenopausalwomen with advanced or metastatic breast cancer,for second-line treatment of postmenopausal patientswith advanced breast cancer who have had disease progressionfollowing tamoxifen therapy, and for adjuvant treatmentof women with early breast cancer. Patients who did not respondto tamoxifen therapy rarely respond to anastrozole.
Biological Activity
Potent and highly selective aromatase (CYP19) inhibitor (IC 50 = 15nM) that has no discernible effect on adrenocorticoid hormone synthesis. Reduces plasma estrogen levels and exhibits antitumor activity in vivo . Orally active.
Biochem/physiol Actions
Anastrozole, which contains a triazole functional group, reversibly binds to the cytochrome P-450 component of aromatase. Binding interferes with the catalytic properties of aromatase, which results in inhibition of estrogen synthesis.
Mechanism of action
Anastrozole, a benzyltriazole derivative, competes with the natural s ubstrate for binding to the active site of the aromatase. The mechanism of enzyme inhibition resides in the coordination of the triazole ring with the hemeiron atom of the aromatase enzyme complex. This coordination ultimately prevents arom atization of androgens into estrogens and, therefore, deprives the tumor of estrogen. This effect is reversible. In the presence of anastrozole, estradiol levels are reduced to undetectable levels, with no adverse effects on levels of any other horm one, including cortisol and aldosterone. Maximal estrogen suppression is produced by a 1mg dose. Estrogen suppression is maintained for up to 6 days after discontinuing anastrozole.
Pharmacokinetics
Anastrozole is well absorbed orally, with biliary elimination as its primary route (85%) and an elimination half-life of approxim ately 50 hours. Approximately 60% of an oral dose is m etabolized in the liver by N-dealkylation, hydroxylation, and glucuronidation to inactive triazole metabolites.
Clinical Use
Anastrozole is a potent and highly selective, nonsteroidal aromatase inhibitor utilized in the treatment of advanced breast cancer that is horm one-responsive. It is considered to be second-line therapy (after tamoxifen) in the treatment of postm enopaus al breast cancer.
Side effects
The most common anastrozole side effects are related to lower estrogen levels in the body. They include hot flashes, nausea and vomiting, and mood changes. Anastrozole could cause your bones to thin, which raises your risk of osteoporosis. It can also cause high cholesterol.
Drug interactions
Potentially hazardous interactions with other drugs
Oestrogen-containing therapies: avoid concomitant
administration as would negate pharmacological
action.
Tamoxifen: avoid concomitant administration.
Environmental Fate
Anastrozole is classified as readily biodegradable and is moderately mobile in soils. The measured octanol-water partition coefficient is low, therefore anastrozole is not predicted to bioaccumulate in aquatic organisms.
Metabolism
Anastrozole is extensively metabolised by postmenopausal women with less than 10% of the dose excreted in the urine unchanged within 72 hours of dosing. Metabolism of anastrozole occurs by N-dealkylation, hydroxylation and glucuronidation via CYP 3A4 and 3A5, and UGT1A4. The metabolites are excreted primarily via the urine. Triazole, the major metabolite in plasma, does not inhibit aromatase.
storage
Store at RT
References
[1] DUKESM. The preclinical pharmacology of “Arimidex” (anastrozole; ZD1033)–a potent, selective aromatase inhibitor.[J]. Journal of Steroid Biochemistry and Molecular Biology, 1996. DOI:10.1016/0960-0760(96)00064-7.
[2] U B. Anastrozole: a new addition to the armamentarium against advanced breast cancer.[J]. American Journal of Clinical Oncology-Cancer Clinical Trials, 1998. DOI:10.1097/00000421-199804000-00014.
[3] L?NNINGP E DowsettM GeislerJ. Pharmacological and clinical profile of anastrozole.[J]. Breast Cancer Research and Treatment, 1998. DOI:10.1023/a:1006000806630.
[4] HOZUMIYASUO. Effects of anastrozole on lipid metabolism compared with tamoxifen in rats.[J]. Breast Cancer Research and Treatment, 2002. DOI:10.1023/a:1020571617274.
Anastrozole Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5978 | 58 |
| Shenzhen Monkono Technology Co.,Ltd | +86-17063441314 | sansbiotech@outlook.com | China | 677 | 58 |
| Wuhan Cell Pharmaceutical Co., Ltd | +86-13129979210 +86-13129979210 | sales@cellwh.com | China | 376 | 58 |
| Doublewin Biological Technology Co., Ltd | +86-19971437628 | doublewin-bella@nandrolonesteroid.com | China | 97 | 58 |
| qingdao future trading Co., Ltd | +86-13335044410 +86-13335044410 | cui56813@163.com | China | 110 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 | Jerry@xhobio.com | China | 737 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
Related articles
- Anastrozole: Pharmacological Properties, Therapeutic Efficacy and Tolerability
- Anastrozole is a potent aromatase inhibitor with superior efficacy and favorable tolerability over tamoxifen in the treatment ....
- Jan 17,2024
- Anastrozole: Applications, pharmacology and side effects
- Anastrozole, a third-generation nonsteroidal aromatase inhibitor, is effective in postmenopausal women with estrogen receptor-....
- Jul 18,2023
- What is Anastrozole?
- Anastrozole, a third-generation nonsteroidal aromatase inhibitor, is effective in postmenopausal women with estrogen receptor-....
- Feb 11,2020
View Lastest Price from Anastrozole manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-28 | Anastrozole ARIMIDEX
120511-73-1
|
US $0.00-0.00 / Bottle | 1Bottle | 99% | 20000 | Shandong Huizhihan Supply Chain Co., Ltd | |
 |
2024-11-28 | Anastrozole ARIMIDEX 1mg Tablets
120511-73-1
|
US $0.00-0.00 / Bottle | 1Bottle | 99% | 20000 | Shandong Huizhihan Supply Chain Co., Ltd | |
 |
2024-11-27 | Anastrozole
120511-73-1
|
US $0.00 / Kg/Bag | 1KG | 98%~102% | 200kg/month | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- Anastrozole ARIMIDEX
120511-73-1
- US $0.00-0.00 / Bottle
- 99%
- Shandong Huizhihan Supply Chain Co., Ltd
-

- Anastrozole ARIMIDEX 1mg Tablets
120511-73-1
- US $0.00-0.00 / Bottle
- 99%
- Shandong Huizhihan Supply Chain Co., Ltd
-

- Anastrozole
120511-73-1
- US $0.00 / Kg/Bag
- 98%~102%
- WUHAN FORTUNA CHEMICAL CO., LTD
120511-73-1(Anastrozole)Related Search:
1of4