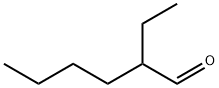
2-ETHYLHEXANAL
- CAS No.
- 123-05-7
- Chemical Name:
- 2-ETHYLHEXANAL
- Synonyms
- 2-Ethylcaproaldehyde;2-ETHYLHEXAL;α-Ethylhexanal;2-ETHYLHEXANAL;2-ethyl-hexana;3-Formylheptane;ETHYLHEXANAL, 2-;Ethylhexaldehyde;hexanal,2-ethyl-;2-Ethylhexan-1-al
- CBNumber:
- CB3241088
- Molecular Formula:
- C8H16O
- Molecular Weight:
- 128.21
- MDL Number:
- MFCD00006987
- MOL File:
- 123-05-7.mol
- MSDS File:
- SDS
| Melting point | -76 °C |
|---|---|
| Boiling point | 55 °C13.5 mm Hg(lit.) |
| Density | 0.822 g/mL at 25 °C(lit.) |
| vapor pressure | 2-2.8hPa at 18.5-23.4℃ |
| refractive index |
n |
| Flash point | 108 °F |
| storage temp. | Flammables area |
| solubility | 0.01g/l |
| form | Liquid |
| color | Clear colorless |
| explosive limit | 1.0-6.6%(V) |
| Water Solubility | Slightly soluble in water. |
| Sensitive | Air Sensitive |
| BRN | 1700556 |
| Stability | Air Sensitive |
| LogP | 3.07 at 25℃ |
| Surface tension | 47.1mN/m at 730mg/L and 20.2℃ |
| CAS DataBase Reference | 123-05-7(CAS DataBase Reference) |
| FDA UNII | SEN8S34O6U |
| EPA Substance Registry System | 2-Ethylhexanal (123-05-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H317-H361 | |||||||||
| Precautionary statements | P201-P210-P280-P302+P352-P308+P313 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 10-36/37/38-43-38-63 | |||||||||
| Safety Statements | 26-36-36/37 | |||||||||
| RIDADR | UN 1191 3/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | MN7525000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29121990 | |||||||||
| Hazardous Substances Data | 123-05-7(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
2-ETHYLHEXANAL price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | E29109 | 2-Ethylhexanal 96% | 123-05-7 | 5ml | $45 | 2024-03-01 | Buy |
| Sigma-Aldrich | 68155 | 2-Ethylhexanal analytical standard | 123-05-7 | 1ml | $74.7 | 2022-05-15 | Buy |
| TCI Chemical | E0125 | 2-Ethylhexanal >95.0%(GC) | 123-05-7 | 25mL | $28 | 2024-03-01 | Buy |
| TCI Chemical | E0125 | 2-Ethylhexanal >95.0%(GC) | 123-05-7 | 500mL | $108 | 2024-03-01 | Buy |
| Alfa Aesar | B23471 | 2-Ethylhexanal, 97% | 123-05-7 | 50g | $29.65 | 2024-03-01 | Buy |
2-ETHYLHEXANAL Chemical Properties,Uses,Production
Description
Ethyl hexaldehyde is a colorless liquid with amild, pleasant odor. Molecular weight = 128.24; Boilingpoint = 163℃; Flash point = 44.4℃; Autoignitiontemperature = 190℃. Explosive limits: LEL = 0.85% at93℃; UEL = 7.2% at 135℃. Hazard Identification (basedon NFPA-704 M Rating System): Health 2, Flammability 2,Reactivity 1. Slightly soluble in water.
Chemical Properties
Ethyl hexaldehyde is a colorless liquid with a mild, pleasant odor.
Chemical Properties
clear liquid
Uses
2-Ethylhexanal may be used as an analytical standard for the determination of the analyte in the atmosphere of charcoal plants by high-performance liquid chromatography and UV detection. It may also be used as an internal standard for the determination of the analyte from wood pellets(6) by chromatography-based techniques.
Uses
2-Ethylhexanal is used as an intermediate for chemical synthesis?and in the production of pharmaceuticals and odorous substances.
Uses
Organic synthesis, perfumes.
Definition
ChEBI: A fatty aldehyde that is heptane in which one of the hydrogens at position 3 has been replaced by a formyl group. It is a metabolite of the plasticisers di-2-ethylhexyl phthalate (DEHP) and di-2-ethylhexyl adipate (DEHA).
Synthesis Reference(s)
Journal of the American Chemical Society, 77, p. 359, 1955 DOI: 10.1021/ja01607a036
Synthesis, p. 767, 1976 DOI: 10.1055/s-1976-24198
General Description
White liquid with a mild odor. Floats on water.
Air & Water Reactions
Spontaneously flammable in air. [Steele and Dugan, Chem. Eng. 66:160(1960)]. Insoluble in water.
Reactivity Profile
2-ETHYLHEXANAL are aldehydes. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.
Hazard
Ignites in air.
Health Hazard
Inhalation may be irritating to mucous membrane; overexposure may cause dizziness and collapse. Ingestion causes irritation of mouth and stomach. Contact with eyes or skin causes irritation.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Mildly toxic by inhalation and skin contact. An eye and severe skin irritant. See also ALDEHYDES. Dangerous fire hazard; spontaneously flammable in air. To fight fire, use foam, CO2, dry chemical, water spray, mist, fog. Incompatible with oxidizing materials. When heated to decomposition it emits acrid and irritating fumes.
Potential Exposure
It is used as a solvent extraction chemical; in organic synthesis; perfume formulation, disinfectant
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with ethyl hexaldehyde you should betrained on its proper handling and storage. Before enteringconfined space where this chemical may be present, checkto make sure that an explosive concentration does not exist.Store in tightly closed containers in a cool, well-ventilatedarea away from oxidizers, strong bases, and combustiblematerials. Metal containers involving the transfer of thischemical should be grounded and bonded. Where possible,automatically pump liquid from drums or other storage containers to process containers. Drums must be equipped withself-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical. Sources of ignition, such as smoking and open flames,are prohibited where this chemical is used, handled, orstored in a manner that could create a potential fire orexplosion hazard. Wherever this chemical is used, handled,manufactured, or stored, use explosion-proof electricalequipment and fittings
Shipping
UN1191 Octyl aldehydes, Hazard Class: 3; Labels: 3-Flammable liquid
Incompatibilities
May form explosive mixture with air. Violent reaction with oxidizers. Incompatible with strong acids; caustics, ammonia, amines. May ignite spontaneously when spilled on clothing or other absorbent materials. May form unstable peroxides on contact with air; under certain conditions ignites spontaneously with air.
Toxics Screening Level
The initial threshold screening level (ITSL) for 2-ethylhexanal is 10 μg/m3 (annual averaging time).
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
2-ETHYLHEXANAL Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8615965530500 | nickzhang@hangyubiotech.com | China | 10983 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21628 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29808 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8772 | 58 |
| Jiangsu Guiren Medicine Co.,Ltd | +86-13815020719 +86-13815020719 | guiren_medicine@163.com | China | 154 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
View Lastest Price from 2-ETHYLHEXANAL manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-22 | 2-ETHYLHEXANAL
123-05-7
|
US $80.00-800.00 / kg | 10kg | 0.99 | 20tons | Zibo Hangyu Biotechnology Development Co., Ltd | |
 |
2020-02-02 | 2-ETHYLHEXANAL
123-05-7
|
US $3.00 / KG | 1KG | 98% | 100KG | Career Henan Chemical Co |
-

- 2-ETHYLHEXANAL
123-05-7
- US $80.00-800.00 / kg
- 0.99
- Zibo Hangyu Biotechnology Development Co., Ltd
-

- 2-ETHYLHEXANAL
123-05-7
- US $3.00 / KG
- 98%
- Career Henan Chemical Co
123-05-7(2-ETHYLHEXANAL)Related Search:
1of4