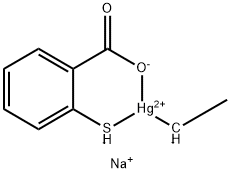
Thimerosal
- CAS No.
- 54-64-8
- Chemical Name:
- Thimerosal
- Synonyms
- THIOMERSAL;MERTHIOLATE;THIMEROSOL;cas no.:54-64-8;MERTHIOLATE SODIUM;elicide;merfamin;merzonin;mertorgan;THIMERSOL
- CBNumber:
- CB4227430
- Molecular Formula:
- C9H9HgNaO2S
- Molecular Weight:
- 404.81
- MDL Number:
- MFCD00013062
- MOL File:
- 54-64-8.mol
- MSDS File:
- SDS
| Melting point | 234-237 °C (dec.)(lit.) |
|---|---|
| Flash point | 250 °C |
| storage temp. | Store at RT. |
| solubility | methanol: 0.1 g/mL, ≤ 10 TE CF |
| form | Powder |
| color | white to off-white |
| PH | 6.0~8.0 (10g/L, 25℃) |
| Water Solubility | 1 G/ML (20 ºC) |
| Merck | 13,9389 |
| BRN | 8169555 |
| Stability | Stable. May degrade in sunlight. Incompatible with strong acids, strong bases, strong oxidizing agents, iodine, heavy metal salts. |
| InChI | InChI=1S/C7H6O2S.C2H4.Hg.Na/c8-7(9)5-3-1-2-4-6(5)10;1-2;;/h1-4,10H,(H,8,9);1H,2H3;;/q;-1;2*+1/p-1 |
| InChIKey | RTKIYNMVFMVABJ-UHFFFAOYSA-L |
| SMILES | O=C1[O-][Hg+2]([CH-]C)[SH-]C2=CC=CC=C12.[Na+] |
| FDA 21 CFR | 310.545 |
| CAS DataBase Reference | 54-64-8(CAS DataBase Reference) |
| EWG's Food Scores | 11-10 |
| FDA UNII | 2225PI3MOV |
| ATC code | D08AK06 |
| EPA Substance Registry System | Thimerosal (54-64-8) |
| UNSPSC Code | 51473202 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H373-H410 | |||||||||
| Precautionary statements | P262-P273-P280-P302+P352+P310-P304+P340+P310-P314 | |||||||||
| Hazard Codes | T+,N,T | |||||||||
| Risk Statements | 26/27/28-33-50/53 | |||||||||
| Safety Statements | 13-28-36-45-60-61-28A | |||||||||
| RIDADR | UN 2025 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OV8400000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29310095 | |||||||||
| Hazardous Substances Data | 54-64-8(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 s.c. in rats: 98 mg/kg (Mason) | |||||||||
| NFPA 704 |
|
Thimerosal price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1659000 | Thimerosal United States Pharmacopeia (USP) Reference Standard | 54-64-8 | 500mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | 71230 | Thimerosal 97.0-101.0% (on dried substance, T) | 54-64-8 | 50G | $681 | 2023-06-20 | Buy |
| Sigma-Aldrich | 71230 | Thimerosal ≥95.0% (Hg) | 54-64-8 | 10g | $152 | 2022-05-15 | Buy |
| Alfa Aesar | J61799 | Thimerosal | 54-64-8 | 10g | $96.1 | 2023-06-20 | Buy |
| Alfa Aesar | J61799 | Thimerosal | 54-64-8 | 25g | $202 | 2023-06-20 | Buy |
Thimerosal Chemical Properties,Uses,Production
Description
Since the 1930s, thimerosal (sodium ethylmercury thiosalicylate) has been used in the manufacture of vaccines to prevent fungal or bacterial contamination of multidose preparations. The single-dose vaccines are much more expensive and thus the use of the preservative significantly reduces the cost of vaccination. There is controversy as to whether the use of thimerosal in vaccines is an etiological factor in autism and other neurodevelopmental disorders such as attention deficit hyperactivity disorder. It has been estimated that children exposed to mercury-containing vaccines could be exposed to levels of mercury far beyond those considered safe by the US Food and DrugAdministration (FDA) standards. Therefore, in 1999, theUS Public Health Service agencies urged vaccine manufacturers to stop or limit the use of the preservative in vaccine formulations. Today, the vaccines on the US Recommended Childhood and Adolescent Immunization Schedule are thimerosal-free although some formulations of the influenza vaccine still contain the mercury-containing preservative. The Centers for DiseaseControl and Prevention (CDC) has discounted an association between exposure to this organic ethylmercury compound and autism. This is based on studies showing that the risk of autism is not increased in children treated with vaccines containing the preservative compared with children who were vaccinated with thimerosal-free formulations. Furthermore, in Denmark, the use of the preservative was discontinued after 1992. After removal of thimerosal, there was a continued rise in new cases of autism.
Chemical Properties
Slightly beige powder
Chemical Properties
Thimerosal is a light cream-colored crystalline powder with a slight, characteristic odor.
Originator
Timeolate,Lifar
Uses
nootropic Anti-Alzheimer’s drug main ingredient of Neupramir derived from Piracetam but 8-30 times more potent
Uses
Thiomersal is an organomercury compound that is used as an antiseptic and antifungal agent It is used as a vaccine preservative. During cell culture, thiomersal is used to prevent overgrowth in fibroblast cells 1. It has been used to preserve Atovaquone 2.
Uses
antifungal, antimicrobial
Uses
Thimerosal is used as ophthalmie preservative; topieal anti-infective; topical veterinary antibacterial and antifungal agent; bacteriostat; fungistat; preservative in vaccines, antitoxins, skin-testing allergens, antiseptics, contact-Iens solutions, and cosmetic products like eye makeup.
Uses
Pharmaceutic aid (preservative).
Definition
ChEBI: An alkylmercury compound (approximately 49% mercury by weight) used as an antiseptic and antifungal agent.
Production Methods
Thimerosal is prepared by the interaction of ethylmercuric chloride, or hydroxide, with thiosalicylic acid and sodium hydroxide, in ethanol (95%).
Manufacturing Process
To a solution or suspension in alcohol of 0.1 mole of methyl mercuric chloride
is added 0.1 mole of sodium hydroxide in water and 0.1 mole of thiosalicylic
acid in ethanol. The product is poured into water, whereupon; the methyl
mercurithiosalicylic acid is precipitated, since it is insoluble in water. This
precipitate can be collected on a filter, and washed well with water to remove
all the alcohol, salts, and free inorganic acids. The washed precipitate may
then be dissolved in a water solution of sodium hydroxide, or, better, in a
water solution of sodium bicarbonate. This produces the water-soluble salt of
the methyl mercurithiosalicylic acid.The methyl mercurithiosalicylic acid is a white solid which melts at about
171°C. It is soluble in alcohol and in ether. It is soluble in either sodium
bicarbonate or sodium hydroxide solution, to form the corresponding salt;
which is suitable for intravenous injection.
The alkali metal salts, such as the sodium and potassium salts, of this acid,
are readily a soluble in water; so are its ammonium salts, and many (probably
all) of its alkyl-ammonium salts; but the alkaline earth salts, such as the
calcium salt, are insoluble in water.
brand name
Thiomersal is INN and BAN;Thiobactal.
Therapeutic Function
Antiseptic, Pharmaceutic aid
General Description
Light cream-colored crystalline powder with a slight odor: pH (1% aqueous solution) 6.7. Slight odor.
Air & Water Reactions
Water soluble.
Reactivity Profile
Thimerosal is incompatible with strong oxidizing agents and strong bases. The oxidation of Thimerosal is greatly accelerated by traces of copper ions. Incompatible with acids, iodine, heavy metal salts and many alkaloids. Can be absorbed by rubber caps. . Stable in air, but not sunlight. May discolor on exposure to light. Dilute aqueous solutions are fairly stable to heat but sensitive to light. Solutions are less stable to heat when acidic than when alkaline. Solutions are most stable to light at pH 5 to 7. Solutions are unstable to heat but not to light in the presence of copper, iron or zinc ions but not in the presence of calcium or magnesium ions.
Hazard
Toxic by ingestion, inhalation.
Fire Hazard
Flash point data for Thimerosal are not available; however, Thimerosal is probably combustible.
Pharmaceutical Applications
Thimerosal has been used as an antimicrobial preservative in
biological and pharmaceutical preparations since the 1930s.
It is used as an alternative to benzalkonium chloride and other
phenylmercuric preservatives, and has both bacteriostatic and
fungistatic activity. Increasing concerns over its safety have,
however, led to questions regarding its continued use in formulations.
Thimerosal is also used in cosmetics and to
preserve soft contact lens solutions.
Therapeutically, thimerosal is occasionally used as a bacteriostatic
and fungistatic mercurial antiseptic, which is usually applied
topically at a concentration of 0.1% w/w. However, its use is
declining owing to its toxicity and effects on the environment.
Contact allergens
Thiomersal is an organic mercury salt prepared by react- ing ethylmercuric chloride (or ethylmercuric hydroxide) with thiosalicylic acid. It is still used as a disinfectant and a preservative agent, but less commonly than previ- ously, especially in contact lens fluids, eyedrops, and vaccines. The ethylmercuric moiety is the major aller- genic determinant, sometimes associated with mercury sensitivity. Thiomersal is an indicator of photosensitiv- ity to piroxicam, through its thiosalicylic moiety.
Safety Profile
Poison by ingestion, subcutaneous, and intravenous routes. Experimental teratogenic and reproductive effects. An eye irritant. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. An ophthalmic preservative, a topical anti-infective, topical veterinary antibacterial and antifungal agent. An FDA over-the-counter drug. When heated to decomposition it emits very toxic fumes of Hg, Na2O, and SOx. See also MERCURY COMPOUNDS.
Safety
Thimerosal is widely used as an antimicrobial preservative in
parenteral and topical pharmaceutical formulations. However,
concern over the use of thimerosal in pharmaceuticals has increased
as a result of a greater awareness of the toxicity of mercury and
other associated mercury compounds. The increasing number
of reports of adverse reactions, particularly hypersensitivity,
to thimerosal and doubts as to its effectiveness as a preservative
have led to suggestions that it should not be used as a preservative in
eye drops or vaccines. In both Europe and the USA,
regulatory bodies have recommended that thimerosal in vaccines be
phased out.
More recent studies assessing the safety of thimerosal in vaccines
have, however, suggested that while the risk of hypersensitivity
reactions is present, the relative risk of neurological harm in infants
is negligible given the quantities of thimerosal present in
vaccines. Regulatory bodies in Europe and the USA have
therefore updated their advice on the use of thimerosal in vaccines
by stating that while it would be desirable for thimerosal not to be
included in vaccines and other formulations the benefits of vaccines
far outweigh any risks of adverse effects associated with their
use.
The most frequently reported adverse reaction to thimerosal,
particularly in vaccines, is hypersensitivity, usually with
erythema and papular or vesicular eruptions. Although not all
thimerosal-sensitive patients develop adverse reactions to vaccines
containing thimerosal, there is potential risk. Patch testing in
humans and animal experiments have suggested that 0.1% w/v
thimerosal can sensitize children. The incidence of sensitivity to
thimerosal appears to be increasing; a study of 256 healthy subjects
showed approximately 6% with positive sensitivity.
Adverse reactions to thimerosal used to preserve contact lens
solutions have also been reported. Reactions include ocular redness,
irritation, reduced lens tolerance, and conjunctivitis. One
estimate suggests that approximately 10% of contact lens wearers
may be sensitive to thimerosal.
Thimerosal has also been associated with false positive reactions
to old tuberculin, ototoxicity, and an unusual reaction to
aluminum in which a patient suffered a burn 5 cm in diameter at
the site of an aluminum foil diathermy electrode after preoperative
preparation of the skin with a 0.1% w/v thimerosal solution in
ethanol (50%). Investigation showed that considerable heat was
generated when such a solution came into contact with aluminum.
An interaction between orally administered tetracyclines and
thimerosal, which resulted in varying extents of ocular irritation,
has been reported in patients using a contact lens solution preserved
with thimerosal.
Controversially, some have claimed a connection between the
use of thimerosal in vaccines and the apparent rise in the incidence
of autism. However, recent studies have shown no association
between thimerosal exposure and autism.
Serious adverse effects and some fatalities have been reported
following the parenteral and topical use of products containing
thimerosal. Five fatal poisonings resulted from the use of 1000 times
the normal concentration of thimerosal in a chloramphenicol
preparation for intramuscular injection.
Ten out of 13 children died as a result of treatment of umbilical
hernia (omphaloceles) with a topical tincture of thimerosal. It
has therefore been recommended that organic mercurial disinfectants
should be restricted or withdrawn from use in hospital since
absorption occurs readily through intact membranes.
In a case of attempted suicide, a 44-year-old man drank
83 mg/kg of a thimerosal-containing solution. Despite spontaneously vomiting after 15 minutes, gastric lavage and administration
of chelating agents on hospital admission, serious symptoms
ultimately ending in coma occurred. The patient survived and after
5 months treatment made a full recovery except for sensory defects
in two toes.
LD50 (mouse, oral): 91 mg/kg
LD50 (rat, oral): 75 mg/kg
LD50 (rat, SC): 98 mg/kg
Environmental Fate
Thimerosal is a cream-colored crystalline powder; it is water soluble and thus the compound can leach into groundwater. Aquatic toxicity has been demonstrated at relatively high concentrations.
storage
Thimerosal is stable at normal temperatures and pressures;
exposure to light may cause discoloration.
Aqueous solutions may be sterilized by autoclaving but are
sensitive to light. The rate of oxidation in solutions is increased by
the presence of trace amounts of copper and other metals. Edetic
acid or edetates may be used to stabilize solutions but have been
reported to reduce the antimicrobial efficacy of thimerosal
solutions.
The solid material should be stored in a well-closed container,
protected from light, in a cool, dry place.
Purification Methods
Recrystallise this antibacterial from EtOH/Et2O. HIGHLY TOXIC. [Trikojus Nature 158 472 1940, Beilstein 10 III 213.]
Toxicity evaluation
Not much is known about the toxic effects of ethylmercury and most toxicologists have assumed that the toxicity is similar to that caused by MeHg. However, because of the differences in the toxicokinetic profiles of the organic mercury salts (see above), this may not be a valid comparison. Methylmercury is a known neurotoxin. However, the neurotoxicity is very complex and depends on the duration of exposure, dose, and the age of the individual. Mercury salts have a strong affinity for thiol groups and this is likely to play a role in mediating their neurotoxicity. Glutathione depletion has been observed after exposure to ethylmercury. Some in vitro studies indicate that oxidative stress leading to lipid peroxidation and DNA damage may play a role in the mechanism of toxicity. Organic mercury salts may exert their toxicity by multiple diverse pathways that have yet to be precisely defined.
Incompatibilities
Incompatible with aluminum and other metals, strong oxidizing
agents, strong acids and bases, sodium chloride solutions,
lecithin, phenylmercuric compounds, quaternary ammonium compounds,
thioglycolate, and proteins. The presence of sodium
metabisulfite, edetic acid, and edetates in solutions can reduce the
preservative efficacy of thimerosal.
In solution, thimerosal may be adsorbed by plastic packaging
materials, particularly polyethylene. It is strongly adsorbed by
treated or untreated rubber caps that are in contact with
solutions.
When it was used with cyclodextrin, the effectiveness of
thimerosal was reduced; however, this was related to the lipid
nature of the other ingredients in the preparation.
Regulatory Status
Included in the FDA Inactive Ingredients Database (IM, IV, and SC injections; ophthalmic, otic, and topical preparations). Included in nonparenteral and parenteral medicines licensed in the UK. In the UK, the use of thimerosal in cosmetics is limited to 0.003% w/w (calculated as mercury) as a preservative in shampoos and haircreams, which contain nonionic emulsifiers that would render other preservatives ineffective. The total permitted concentration (calculated as mercury) when mixed with other mercury compounds is 0.007% w/w. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Thimerosal Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Rochi Pharmaceutical Co.,Ltd. | 21-38751876 +8615000076078 | info@rochipharma.com | China | 431 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | FandaChem@Gmail.com | China | 3966 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29808 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10244 | 58 |
View Lastest Price from Thimerosal manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-01-08 | THIOMERSAL
54-64-8
|
US $23.00 / kg | 1kg | 99.96% | 50kg | Wuhan Boyuan Import & Export Co., LTD | |
 |
2021-11-15 | Thimerosal
54-64-8
|
US $25.00 / KG | 1KG | 99% | 5ton/Month | Qiuxian Baitai New Material Co., LTD | |
 |
2020-05-04 | Thimerosal
54-64-8
|
US $0.00-0.00 / KG | 1KG | 99.0% | 600 Tons | Shaanxi Dideu Medichem Co. Ltd
|
-

- THIOMERSAL
54-64-8
- US $23.00 / kg
- 99.96%
- Wuhan Boyuan Import & Export Co., LTD
-

- Thimerosal
54-64-8
- US $25.00 / KG
- 99%
- Qiuxian Baitai New Material Co., LTD
-

- Thimerosal
54-64-8
- US $0.00-0.00 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd