Pazopanib Hydrochloride
- CAS No.
- 635702-64-6
- Chemical Name:
- Pazopanib Hydrochloride
- Synonyms
- Pazopanib hydrochloride;Pazopanib HCl;ArMala;786034;CS-462;GW-786034B;GW786034 HCl;Votrient HCl;Pazopanib HCI;Unii-33Y9anm545
- CBNumber:
- CB42495435
- Molecular Formula:
- C21H24ClN7O2S
- Molecular Weight:
- 473.98
- MDL Number:
- MFCD12546138
- MOL File:
- 635702-64-6.mol
- MSDS File:
- SDS
| Melting point | >290°C (dec.) |
|---|---|
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | Acetonitrile (Slightly), DMSO (Slightly) |
| form | Yellow powder. |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | MQHIQUBXFFAOMK-UHFFFAOYSA-N |
| SMILES | CC1N(N=C2C=C(N(C3C=CN=C(NC4C=CC(C)=C(S(=O)(=O)N)C=4)N=3)C)C=CC=12)C.Cl |
| NCI Dictionary of Cancer Terms | pazopanib hydrochloride |
| FDA UNII | 33Y9ANM545 |
| NCI Drug Dictionary | pazopanib hydrochloride |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H332-H335 |
| Precautionary statements | P261-P280-P305+P351+P338 |
Pazopanib Hydrochloride price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML3076 | Pazopanib hydrochloride ≥98% (HPLC) | 1149669-28-2 | 10MG | $86.1 | 2022-05-15 | Buy |
| Sigma-Aldrich | SML3076 | Pazopanib hydrochloride ≥98% (HPLC) | 1149669-28-2 | 50MG | $348 | 2022-05-15 | Buy |
| ChemScene | CS-0126 | PazopanibHydrochloride 99.84% | 635702-64-6 | 500mg | $420 | 2021-12-16 | Buy |
| ApexBio Technology | A8347 | PazopanibHydrochloride | 635702-64-6 | 500mg | $480 | 2021-12-16 | Buy |
| ChemScene | CS-0126 | PazopanibHydrochloride 99.84% | 635702-64-6 | 1g | $552 | 2021-12-16 | Buy |
Pazopanib Hydrochloride Chemical Properties,Uses,Production
Description
Pazopanib Hydrochloride is the hydrochloride salt of a small molecule inhibitor of multiple protein tyrosine kinases with potential antineoplastic activity. It is an oral second-generation multitarget TKI developed by GSK and approved for marketing by the FDA in 2009 and the EMA in 2010. It targets the VEGFR, platelet-derived growth factor receptor, and c-kit, key proteins responsible for tumor growth and survival. It is used to treat patients with advanced RCC and advanced soft tissue sarcoma who have experienced chemotherapy. Pazopanib Hydrochloride has a role as an antineoplastic agent, a vascular endothelial growth factor receptor antagonist, a tyrosine kinase inhibitor, and an angiogenesis-modulating agent.
Originator
GlaxoSmithKline (US)
Uses
Pazopanib Hydrochloride (GW786034) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively - See more at: http://www.selleckchem.com/products/Pazopanib-Hyd
Definition
Pazopanib Hydrochloride is used for treatment of kidney cancer. Pazopanib is the latest VEGFR kinase inhibitor to reach the market. It is indicated for the oral treatment of advanced RCC. The biological functions of the VEGF family are mediated by activation of three structurally homologous tyrosine kinase receptors, VEGFR-1, VEGFR-2, and VEGFR3.
brand name
Votrient
Clinical Use
Pazopanib is a potent and selective multi-targeted receptor tyrosine kinase inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-a/b, and c-kit that blocks tumor growth and inhibits angiogenesis. It was approved for renal cell carcinoma by the U.S. Food and Drug Administration in 2009 and is marketed under the trade name Votrient by the drug’s manufacturer, GlaxoSmithKline.
Side effects
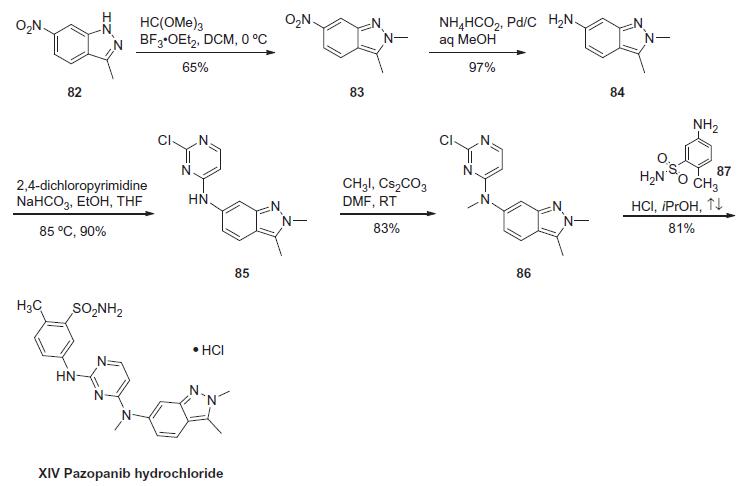
Pazopanib is synthesized in five chemical steps starting from 3-methyl-6-nitroindazole, which is converted to the corresponding 2,3-dimethylindazole analog via N-methylation with trimethyloxonium tetrafluoroborate. Subsequent reduction of the nitro group to the amino group using tin chloride followed by condensation with 2,4dichloropyrimidine yields a chloropyrimidinylaminoindazole intermediate. The final two steps leading up to pazopanib consist of an N-methylation reaction using iodomethane and cesium carbonate followed by condensation with 5-amino-2-methylbenzenesulfonamide.
Synthesis
The synthesis of pazopanib begins with methylation of 3-methyl-6- nitroindazole (82) with trimethyl orthoformate in the presence of BF3?¤OEt to give indazole 83 in 65% yield. Reduction of the nitro group was achieved via transfer hydrogenation to give 84 in 97% yield, and this was followed by coupling the aniline with 2,4-dichloropyrimidine in a THF-ethanol mixture at elevated temperature to provide diarylamine 85 in 90% yield. The aniline nitrogen was then methylated using methyl iodide to give 86 in 83% yield prior to coupling with 5-amino-2-methylbenzenesulfonamide (87) and salt formation using an alcoholic solution of HCl to furnish pazopanib hydrochloride (XIV) in 81% yield.
target
VEGFR1
References
[1] Sodeifian, G. et al. “Solubility of pazopanib hydrochloride (PZH, anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling.” The Journal of Supercritical Fluids 55 1 (2022): 0.
[2] “Stability Indicating HPTLC Method Development and Validation for the Estimation of Pazopanib Hydrochloride in Bulk and its Dosage Form.” International Journal of Pharmaceutical Research 18 1 (2020).
[3] K. Kawasaki . “Retrospective Safety Analysis in Advanced Soft Tissue Sarcoma Patients of Pazopanib Hydrochloride.” Annals of Oncology 24 (2013): Page ix38.
[4] Gupta, Amit and Rashmi Dahima. “Application of Simplex Lattice Mixture design and desirability function in the development and Optimization of SEDDS for protein kinase inhibitor-Pazopanib Hydrochloride.” Research Journal of Pharmacy and Technology 83 1 (2023): 0.
Pazopanib Hydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15350 | 58 |
| BIONNA MEDICINE CO.,LTD | 01056380788-8515; +8618518759099 | 790226113@qq.com | China | 52 | 58 |
| Yangzhou Qinyuan Pharmatech Co.,ltd | +86-18752526868 | jennysun@yzqyyykj.com | China | 81 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 485 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| Anqing Chico Pharmaceutical Co., Ltd. | 15380796838 | chloewu@chicopharm.cn | CHINA | 340 | 58 |
| Lianyungang happen teng technology co., LTD | 15950718863 | wang666xt@163.com | CHINA | 295 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29860 | 58 |
Related articles
- Synthesis, Detection and Bioactivity of Pazopanib Hydrochloride
- Pazopanib Hydrochloride is an oral angiogenesis inhibitor targeting VEGFR and PDGFR.
- Sep 13,2022
Related Qustion
- Q:What is the solubility of Pazopanib hydrochloride?
- A:Pazopanib Hydrochloride was found to exhibit a low aqueous solubility. The intestinal permeability of PZH is considered to be ....
- Mar 19,2024
View Lastest Price from Pazopanib Hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-01-13 | Pazopanib Hydrochlorid
635702-64-6
|
US $0.00 / g | 1g | More Than 99% | 100kg/Month | BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | |
 |
2024-11-26 | Pazopanib hydrochloride
635702-64-6
|
US $0.00-0.00 / kg | 1kg | 99%,single impurity<0.1 | 1 ton | Nanjing Fred Technology Co., Ltd | |
 |
2024-11-18 | Pazopanib Hydrochloride
635702-64-6
|
US $48.00-143.00 / mg | 99.69% | 10g | TargetMol Chemicals Inc. |
-

- Pazopanib Hydrochlorid
635702-64-6
- US $0.00 / g
- More Than 99%
- BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
-

- Pazopanib hydrochloride
635702-64-6
- US $0.00-0.00 / kg
- 99%,single impurity<0.1
- Nanjing Fred Technology Co., Ltd
-

- Pazopanib Hydrochloride
635702-64-6
- US $48.00-143.00 / mg
- 99.69%
- TargetMol Chemicals Inc.
635702-64-6(Pazopanib Hydrochloride)Related Search:
1of4





