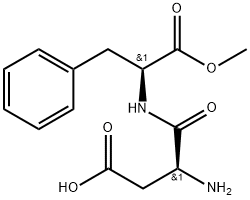
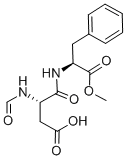
Aspartame
- CAS No.
- 22839-47-0
- Chemical Name:
- Aspartame
- Synonyms
- APM;ASPARTAME POWDER;ASP-PHE METHYL ESTER;ASPARTAM;(S)-3-AMino-4-(((S)-1-Methoxy-1-oxo-3-phenylpropan-2-yl)aMino)-4-oxobutanoic acid;Asp-Phe-OMe;Glycopeptide;Equal;Protein sugar;ASPARTAME-ACESULFAMESALT
- CBNumber:
- CB4345527
- Molecular Formula:
- C14H18N2O5
- Molecular Weight:
- 294.31
- MDL Number:
- MFCD00002724
- MOL File:
- 22839-47-0.mol
- MSDS File:
- SDS
| Melting point | 242-248 °C |
|---|---|
| alpha | 15.5 º (c=4, 15N formic acid) |
| Boiling point | 436.08°C (rough estimate) |
| Density | 1.2051 (rough estimate) |
| refractive index | 14.5 ° (C=4, 15mol/L Formic Acid) |
| storage temp. | 2-8°C |
| solubility | Sparingly soluble or slightly soluble in water and in ethanol (96 per cent), practically insoluble in hexane and in methylene chloride. |
| pka | pKa 3.19±0.01 (H2O t=25.0 I=0.100(NaCl))(Approximate);7.87±0.02(H2O t=25.0 I=0.100(NaCl))(Approximate) |
| form | Powder |
| color | White |
| Odor | odorless with a sweet taste |
| PH | pH(8g/l, 25℃) : 4.5~6.0 |
| Water Solubility | Soluble in formic acid, dimethyl sulfoxide. Sparingly soluble in water and ethanol. |
| Merck | 14,839 |
| BRN | 2223850 |
| Sequence | H-Asp-Phe-OMe |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | IAOZJIPTCAWIRG-QWRGUYRKSA-N |
| LogP | 0.542 (est) |
| FDA 21 CFR | 172.804 |
| Substances Added to Food (formerly EAFUS) | ASPARTAME |
| CAS DataBase Reference | 22839-47-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | glycopeptide |
| FDA UNII | Z0H242BBR1 |
| EPA Substance Registry System | L-Phenylalanine, L-.alpha.-aspartyl-, 2-methyl ester (22839-47-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280-P304+P340-P305+P351+P338 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | WM3407000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29242990 | |||||||||
| Toxicity | TDLo orl-wmn: 3710 mg/kg:SKN AIMEAS 104,207,86 | |||||||||
| NFPA 704 |
|
Aspartame price More Price(63)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | WH0010461M1 | Monoclonal Anti-MERTK antibody produced in mouse clone 2D2, purified immunoglobulin, buffered aqueous solution | 22839-47-0 | 100μG | $524 | 2024-03-01 | Buy |
| Sigma-Aldrich | A5139 | Asp-Phe methyl ester ≥98% | 22839-47-0 | 1g | $76.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 47135 | Aspartame analytical standard | 22839-47-0 | 500mg | $33.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1043706 | Aspartame United States Pharmacopeia (USP) Reference Standard | 22839-47-0 | 200mg | $491 | 2024-03-01 | Buy |
| TCI Chemical | A0997 | Aspartame >98.0%(HPLC)(T) | 22839-47-0 | 1g | $41 | 2024-03-01 | Buy |
Aspartame Chemical Properties,Uses,Production
Artificial sweeteners
Aspartame is a kind of artificial sweeteners, belongs to the amino acid dipeptide derivatives, by the chemist developed ulcer drugs found in 1965. With low dosage, high sweetness (sweetness is 150 to 200 times of sucrose), good taste, enhance flavor of citrus and other fruits and reducing heat does not produce dental caries, toxicity than saccharin and other synthetic sweetening agent advantages, is widely applied to beverages, diabetic food and some slimming health food, our daily life to drink cola formula once containing the product.
Aspartame in the metabolic processes in the body and the main degradation products are phenylalanine, methanol and aspartic acid, does not enter blood circulation, and does not accumulate in the body, food for the health harmless. But due to metabolic defects in patients with phenylketonuria (PKU), excessive body phenylalanine can influence its development, so in patients with the disease to disable adding aspartame.
Neotame
Neotame is aspartate dipeptide derivatives, is a new product developed at a cost of $80 million by American newt company after aspartame, representing the latest achievements of sweetener research field. It is according to the human sweet receptor double hydrophobic binding hypothesis. In aspartame molecule with a hydrophobic groups and the formation of aspartame derivatives. It can also act on the human sweet receptor of two hydrophobic binding sites and therefore sweetness increased greatly, 6000 to 10000 times sweeter than sucrose, 30 to 60 times than Biasiba sweet .
It retains many excellent characteristics such as aspartame, pure sweet taste and good flavor enhancing properties distribution, no energy, no caries, stable in acidic medium. Moreover, it is still a lot better than aspartame in dry conditions, it has a longer shelf life; in neutral medium or instantaneous high temperature sterilization conditions, its stability greatly exceed aspartame, which can be used as a sweetener in baking. Neotame can also be used together with reducing sugar and aldehyde flavor without adverse reaction, its safety is better than aspartame and has been greatly improved. Due to its high sweetness, etc. Sweeter is lower than the cost of aspartame. Therefore, neotame has huge market potential. In December of 1998, neotame as food sweeteners status of application have been proposed in the United States, and some other countries for the certification work is in July 9, 2002 . The U. S. Food and Drug Administration (FDA) confirmed the neotame safety and functional type, currently is in March 10, worldwide more regulatory agency and national review in 2003, China's Ministry of Health approval for neotame as a sweetener used in all kinds of food.
The above information is edited by the Chemicalbook Han Ya.
Content analysis
Accurately take sample of about 300 mg, moves into a 150ml beaker, dissolve in 96% formic acid 1.5ml, and glacial acetic 60ml. with crystal violet solution (ts-74), with 0.1 mol/L HClO4 immediately titrate to the end of green. At the same time a blank titration and make necessary correction. Each mI.0.1mol/L perchloric acid is the equivalent of 29.43mg C14H18N2O5.
Toxicity
Entering the human body can quickly metabolic day aspartic acid and phenylalanine, two kinds of amino acids are absorbed, which does not accumulate in the organization. But phenylketonuria patients cannot use. Therefore it is necessary to specially marked. Every year in China about 1500~2000 benzene acetone urine disease in children born, after eating can be in vivo abnormal accumulation caused by brain damage, mental development retardation and epilepsy.
The ADI value is 0~40mg/kg (FAO/WHO, 2001). Contains two ketone piperazine ADI value is 0~7.5.
GRAS (FDA, 172.804,2000).
Use limits
GB 2760-2001: all types of food (except canned food), are limited to GMP.
FAO/WHO (1984): sweets 0.3%, gum 1.0%; beverage 0.1%, 0.5% of breakfast cereals, and used for the preparation of diabetes, hypertension, obesity, cardiovascular patients with low sugar, low calorie health food, dosage depends on the need to set. Can also be used as flavor enhancer.
chemical property
White crystalline powder, odorless, there is strong sweet, sweet and pure, sweetness of sucrose was 100-200 times. Melting point is 235℃ (decomposition). Amino acids is with the general nature. In dry conditions or pH value of 2 to 5 range, it is stability, strong acidic water solution can hydrolyzed to produce amino acid monomer, in neutral or alkaline conditions it can be cyclized to diketopiperazine. Solubility in water (25℃) is relevant to pH value, pH 7.0 was 10.2%, pH value 3.72 is 18.2%. At 25, isoelectric point is pH value of 5.2. Mice by oral LD50 > 10 g/kg Adl is 0~40mg/kg (FAO/who, 1994)
Uses
1.Asparagus sweet is artificial synthesis of low calorie sweeteners, often used with sugar or other sweeteners. It can be used for all kinds of food, according to the production need to use, the general dosage is 0.5g/kg.
2.It Is used as a food additive, high sweetness nutritive sweeteners.
3.Non nutritive sweeteners. Flavoring agents.
4.According to China GB2760-90 provisions for all kinds of food, the maximum amount of use as normal production needs. According to the FAO/WHO (1984) provisions for sweets, dosage of 0.3%, 1.0% gum, beverage 0.1%, 0.5% of breakfast cereals, and used for the preparation of diabetes, hypertension, obesity, cardiovascular patients with low sugar, low calorie health food, dosage depends on the need to set. Can also be used as a flavor enhancer.
Aspartame is a L-aspartic acid and L-phenylalanine (body needed nutrients) two peptide synthesis, can be completely absorbed by the human body metabolism, non-toxic harmless, safe and reliable, cool and refreshing taste like sugar, but is 200 times sweeter than sucrose, the heat is only 1/200 sucrose, eat no gingivae that does not affect the blood glucose, obesity, hypertension, coronary heart disease. The World Health Organization (WHO) and the United Nations Food and Agriculture Organization (FAO) identified as A (1) level of sweetener, has been in the world more than 130 countries and regions approved for use. Widely added in a variety of food, non-staple food and all kinds of hard and soft drinks, the use of aspartame has more than 4000 kinds of varieties. Can be used as food additives, high sweetness sweeteners with nutrition. Packing: 25 kg fibreboard drum, lined with plastic bag.
Methods of production
1. By L-aspartate and L-phenylalanine methyl ester condensation.
2. By L-aspartic acid and L-phenylalanine methyl ester hydrochloride condensation.
There are two kinds of synthetic and enzymatic synthesis.
enzymic synthesis :
Department of chemistry, Wuhan University, Tao Guoliang, gave the following synthetic route:
The preparation of I : 0.5mmol benzyloxy carbonyl aspartic acid, 1.5mmol phenylalanine methyl ester hydrochloride and 2.5ml water were added to 25 ml Erlenmeyer flask, with ammonia to adjust the pH to 6, adding 7mg of thermophilic protease, at 40℃mixing reaction for 6h. Filter and washed with distilled water, and drying to obtain white solid (I) 0.29g, 95.6% yield, melting point is 116 to 118 ℃. Elemental analysis results: C 62.96%, H6.09%.N of 6.65%.
preparation of II: it will be joined the conical flasks 0.5g sample I and 20mL3mol/L hydrochloride 25ml and 45℃ in the mixing reaction for 0.5h. Filtration and washing with distilled water, drying to obtain 0.32g the product II, yield is 92%, melting point is 129 to 131℃. Elemental analysis results: C 61.45%, H 5.42% , N 6.82%.
Preparation of III will 0.2g palladium carbon catalyst (10%), 20 ml of glacial acetic acid, 5 ml of water add 100 ml three necked flask, hydrogenation activation 1.5h. Join 0.6g II 20 ml acetic acid dissolution and, at 30℃ stirring hydrogenated after 6h. the reaction is completed after filtration, catalyst with acetic acid washing 3 times; the filtrate and washings were concentrated under reduced pressure to dry, 15ml of benzene, continue to decompress and concentrate to dry white solid, and drying to obtain 0.38g the product III, and the yield was 92.3%, the melting point is 245 ℃. The elemental analysis results: C 55.63%, H6.23%, N 8.96%.
Chemical synthesis method
The aspartic acid and phenylalanine as raw material, by amino protection, anhydride, condensation, hydrolysis, neutralization and other steps of the synthesis. Different protecting groups, different methylated sequence can have a variety of different synthesis methods. Such as the use of formyl as protecting groups and after methyl esterification process route.Into a 250ml flask into 27mL 95% of the methanol and 0.2g oxidation of magnesium and magnesium oxide is dissolved, add 100ml 98% of acetic anhydride, at this time, the temperature gradually rose to 40 ℃, adding acid 67gL-aspartic, heated to 50℃, stirring reaction time 2.5h insulation and fill with 15mL98% acetic anhydride, temperature and reaction time 2.5h, join 16ml isopropanol and continue for 1.5h, the reaction after cooling to room temperature.
Put the inner anhydride materialized to join 1000ml flask, add 207mL ethyl acetate and 66Gl-phenylalanine, stirring 1.5h in 25 to 30℃, add the glacial acetic acid 126mL, continue to reflect 4.5h, the reaction after the end of the vacuum to remove the solvent and to the temperature of the reaction system 65℃ so far. Then add 35% hydrochloric acid methylmercury, heating to 60 ℃, return at the end of the reaction of the hydrolysis of 2h, atmospheric distillation until boiling temperature of 63℃ (reaction temperature of 73 degrees C) so far, adding methanol 180, to continue the distillation to the system temperature of 85 ℃ so far. After cooled to 25℃, the removal of the vacuum light group.
Adding 35% hydrochloric acid 54mL to the hydrolysis liquid, methanol 9mL and water 43mL. In 20 to 30℃for esterification reaction was then filtered, washed separation from α-APM hydrochloride. It dissolves in 600ml distilled water, to 40 to 50 ℃ of 5%~10% NaOH solution and to Ph=4.5. cooling below, filtering, washing to α-APM crude product, and then dissolved in 500 ml of methanol and water (volume ratio of 1:2) mixture. The cooling crystallization, filtration washing, vacuum drying 45% yield in terms of L-phenylalanine.
The Japanese scholars put forward a route without protection:
90g phenylalanine methyl ester hydrochloride is dissolved in 450mL water, 24g sodium carbonate neutralization, two vinyl chloride extraction and 2350mL obtained. Adding 9g phenylalanine methyl ester of acetic acid and methanol extracts 8mL, 15.2g aspartic anhydride hydrochloride added at-20 deg.c, stirring for 30min, hot water and sodium carbonate were added to 70~80 350mL C (5.7g) 300mL. solution with 150mL two vinyl chloride 2 remaining after extraction of phenylalanine methyl ester, water with dilute hydrochloric acid to adjust the Ph value to the 4.8. of the aqueous solution of paper electrophoresis measured with 18.2G (molar yield of 60%) and 6.1g (α-APM beta 20% molar yield) β--APM. This solution is vacuum concentration 100mL, plus.36% hydrochloric acid 30mL, set the refrigerator overnight. A-APM-HCl 21.3g Precipitation Crystallization (yield 58%), the crystallization of filtered and dissolved in 200mL water solution. Stirring at 50 ℃, with 5% sodium carbonate solution to adjust the Ph value to 4.8, and then place the refrigerator overnight in the analysis And filtering to obtain alpha APM crystallization) (43% yield). Crystal dissolved in 500ml water. In 45℃ by Dowex 1 x 4 (acetate) columns (1 x 20cm), and 20 ml of water flushing, effluent and washings together vacuum concentration, precipitation of alpha APM crystallization 11.2g. yield of 37%, melting point 235~236℃ (decomposition), than the rotation alpha D22 32.0 ℃ = 1, Cu Suanzhong. Elemental analysis results: 55.30% C, H 6.19%, n 9.36%.
Category
Toxic substances
Explosive hazard characteristics
Edible contact dermatitis.
Combustible hazard characteristics
Combustible; combustion produces toxic nitrogen oxide smoke
Storage and transportation characteristics
Combustible; combustion produces toxic nitrogen oxide smoke
Fire extinguishing agent
Dry powder, foam, sand, carbon dioxide, mist water
Description
Aspartame is a synthetic non-caloric sweetener that is metabolized to phenylalanine, aspartic acid, and methanol in the gut. Aspartame (80 mg/kg per day for 90 days) increases plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity, induces hepatocyte degeneration and leukocyte infiltration in the liver, and reduces hepatic levels of reduced glutathione (GSH), oxidized glutathione (GSSG), and γ-glutamylcysteine (γ-GC) in mice. Formulations containing aspartame have been used as sweetening agents and flavor enhancers in foods and beverages.
Description
Aspartame is the most popular artificial sweetener in the United States. It is sold as sweeteners such as NutraSweet and Equal, but it is also incorporated into thousands of food products.
Chemical Properties
Aspartame has no odor, but has an intense sweet taste. It is a high intensity sweetener, about 160 to 200 times sweeter than sucrose. Normal digestive processes convert aspartame to phenylalanine, aspartic acid and methanol. Metabolism of aspartame in the body provides approximately 17 kJ (4 kcal)/g. The stability of aspartame is affected by moisture, pH and temperature. For a detailed description of this compound, refer to Burdock (1997).
Chemical Properties
white powder or tablets
Chemical Properties
Aspartame occurs as an off white, almost odorless crystalline powder with an intensely sweet taste.
Chemical Properties
Aspartame (N-L-aspartyl-L-phenylalanine-1-methyl ester, 3-amino-N-(a-carbomethoxy-
phenethyl)-succinamic acid-N-methyl ester) is an intense sweetener widely
used in foods and beverages. Its solubility in water is approximately 10 g/L at room
temperature. Aspartame is not fully stable under common processing and storage
conditions of foods and beverages with the highest stability around pH 4.3.
Aspartame is about 200 times sweeter than sucrose with a clean, but slightly
lingering sweetness. It is used as the single sweetener, but often also in blends with other intense sweeteners owing to synergistic taste enhancement and taste quality
improvement often seen in such blends.
In the European Union, aspartame is approved as E 951 for a large number of
food applications. In the United States, it is approved as a multipurpose sweetener
for food and beverage uses and it is also approved in many other countries.
Originator
Canderel,Searle,France,1979
History
Aspartame was discovered accidentally in 1965 during a search for drugs to treat gastric ulcers. James M. Schlatter, an organic chemist working for G. D. Searle & Company, was using aspartyl-phenylalanine methyl ester (aspartame) in a synthesis procedure and inadvertently got some of the compound on his hands.
Uses
Aspartame in powder form for limited uses such as cereals, powdered drinks, and chewing gum. When aspartame is used in baked goods and baking mixes, it should not exceed 0.5% by weight. Packages of the dry, free-fl owing aspartame are required to prominently display the sweetening equivalence in teaspoons of sugar.
Uses
A dipeptide ester about 160 times sweeter than sucrose in aqueous solution. A non-nutritive sweetener.
Uses
The chemical name for aspartame is L-aspartyl-L-phenylalamine methyl
ester.
It is a white crystalline powder and is about 200 times as sweet as
sucrose. It is noted for a clean, sweet taste that is similar to that of
sucrose.
Aspartame is the most widely used artificial sweetener in the world.
It was approved by the FDA for use in the USA in 1981, and now is
approved for use in several other countries of the world. One of the
drawbacks of aspartame is its instability to heat and acid. Under acidic
conditions aspartame slowly hydrolyzes leading to a loss of sweetness,
chemical interaction, and microbial degradation. The shelf life of the
aspartame-sweetened products with high water content is limited to
about 6 months, after which it breaks down into its constituent components
and loses its sweetening abilities. At elevated temperatures, solid
aspartame slowly releases methanol to form aspartyl phenylalamine
and the dioxopiperazine. This reaction is especially favored at neutral and
alkaline pH values. Because of this reason, aspartame cannot be used
in hot baking foods.
Another disadvantage of aspartame was noticed in the human digestive
system. When the body ingests aspartame, it breaks down into its
three constituent components: phenylalamine, aspartate, and methanol.
The phenylalamine and aspartate are handled by enzymes in the stomach
and in the small intestine, while the methanol is transported to the
liver for detoxification. The metabolism of phenylalamine requires an
enzyme that is not produced by a small proportion of the population
having a genetic disorder called phenyl keton uria (PKU). Aspartame
should be avoided by persons suffering from PKU. A warning to PKU sufferers
on aspartame-containing products is required in many countries.
Uses
Aspartame is a high-intensity sweetener that is a dipeptide, provid- ing 4 cal/g. it is synthesized by combining the methyl ester of phenylalanine with aspartic acid, forming the compound n-l-alpha- aspartyl-l-phenylalanine-1-methyl ester. it is approximately 200 times as sweet as sucrose and tastes similar to sugar. it is compara- tively sweeter at low usage levels and at room temperature. its mini- mum solubility is at ph 5.2, its isoelectric point. its maximum solubility is at ph 2.2. it has a solubility of 1% in water at 25°c. the solubility increases with temperature. aspartame has a certain insta- bility in liquid systems which results in a decrease in sweetness. it decomposes to aspartylphenylalanine or to diketropiperazine (dkp) and neither of these forms is sweet. the stability of aspartame is a function of time, temperature, ph, and water activity. maximum stability is at approximately ph 4.3. it is not usually used in baked goods because it breaks down at the high baking temperatures. it contains phenylalanine, which restricts its use for those afflicted with phenylketonuria, the inability to metabolize phenylalanine. uses include cold breakfast cereals, desserts, topping mixes, chew- ing gum, beverages, and frozen desserts. the usage level ranges from 0.01 to 0.02%.
Uses
Non-nutritive sweetener.
Definition
ChEBI: A dipeptide composed of methyl L-phenylalaninate and L-aspartic acid joined by a peptide linkage.
Production Methods
Aspartame is produced by coupling together L-phenylalanine (or Lphenylalanine methyl ester) and L-aspartic acid, either chemically or enzymatically. The former procedure yields both the sweet aaspartame and nonsweet β-aspartame from which the α-aspartame has to be separated and purified. The enzymatic process yields only α-aspartame.
Production Methods
Aspartame is synthesized using the L enantiomer of phenylalanine. The L enantiomer is separated from the D enantiomer, the racemic mixture, by reacting it with acetic anhydride (CH32
Preparation
By coupling the amino acids L-phenylalanine and L-aspartic acid, and the esterification of the carboxyl group of the phenylalanine moiety to produce the methyl ester. This esterification can occur before or after coupling. The crystallized slurry is centrifuged and the resulting “wet-cake” is washed to remove impurities.
Manufacturing Process
A solution of 88.5 parts of L-phenylalanine methyl ester hydrochloride in 100
parts of water is neutralized by the addition of dilute aqueous potassium
bicarbonate, then is extracted with approximately 900 parts of ethyl acetate.
The resulting organic solution is washed with water and dried over anhydrous
magnesium sulfate. To that solution is then added 200 parts of Nbenzyloxycarbonyl-
L-aspartic acid α-p-nitrophenyl, β-benzyl diester, and that
reaction mixture is kept at room temperature for about 24 hours, then at
approximately 65°C for about 24 hours. The reaction mixture is cooled to
room temperature, diluted with approximately 390 parts of cyclohexane, then
cooled to approximately -18°C in order to complete crystallization. The
resulting crystalline product is isolated by filtration and dried to afford β-
benzyl N-benzyloxycarbonyl-L-aspartyl-L-phenylalanine methyl ester, melting
at about 118.5-119.5°C.
To a solution of 180 parts of β-benzyl N-benzyloxycarbonyl-L-aspartyl-Lphenylalanine
methyl ester in 3,000 parts by volume of 75% acetic acid is
added 18 parts of palladium black metal catalyst, and the resulting mixture is
shaken with hydrogen at atmospheric pressure and room temperature for
about 12 hours. The catalyst is removed by filtration, and the solvent is
distilled under reduced pressure to afford a solid residue, which is purified by
recrystallization from aqueous ethanol to yield L-aspartyl-L-phenylalanine
methyl ester. It displays a double melting point at about 190°C and 245-
247°C.
Therapeutic Function
Sugar supplement
Biotechnological Production
Aspartame is produced from L-aspartic acid and L-phenylalanine and methanol or
alternatively L-phenylalanine methyl ester. The standard process uses common
chemical methods of peptide synthesis. Enzymatic coupling of the two amino
acids is also possible. N-formyl-L-aspartic acid and L- or D.L-phenylalanine methyl
ester can be condensed to aspartame by thermolysin-like proteases. The
formylated aspartame can be deformylated chemically or with a formylmethionyl
peptide deformylase to yield the sweetener.The enzymatic coupling does not
require L-phenylalanine but can start from the racemic product obtained in
chemical synthesis, and the remaining D-phenylalanine can be racemized again.
Production processes based on fermentation are available for the two main
components, aspartic acid and phenylalanine.
General Description
Asp-Phe methyl ester (aspartame, APM, ASP), a dipeptide ester, is made up of phenyl alanine and aspartic acid. Its genotoxic effects have been investigated. Its interaction with certain hydrocolloids has been studied.
Pharmaceutical Applications
Aspartame is used as an intense sweetening agent in beverage
products, food products, and table-top sweeteners, and in
pharmaceutical preparations including tablets, powder mixes,
and vitamin preparations. It enhances flavor systems and can be
used to mask some unpleasant taste characteristics; the approximate
sweetening power is 180–200 times that of sucrose.
Unlike some other intense sweeteners, aspartame is metabolized
in the body and consequently has some nutritive value: 1 g provides
approximately 17 kJ (4 kcal). However, in practice, the small
quantity of aspartame consumed provides a minimal nutritive
effect.
Biochem/physiol Actions
Asp-Phe methyl ester (Asp-Phe-OMe) is used as a synthetic sweeter, sugar substitute. Asp-Phe methyl ester is being studied for a variety of potential benefits as a nutrition supplement, such as the delay of osteoarthritis and modulation of rheumatoid factor activity. Asp-Phe methyl ester is being studied for its effect on thrombin activity and blood clotting.
Safety Profile
Human systemic effects byingestion: allergic dermatitis. Experimental reproductiveeffects. When heated to decomposition it emits toxicfumes of NOx.
Safety
Aspartame is widely used in oral pharmaceutical formulations,
beverages, and food products as an intense sweetener, and is
generally regarded as a nontoxic material. However, the use of
aspartame has been of some concern owing to the formation of the
potentially toxic metabolites methanol, aspartic acid, and phenylalanine.
Of these materials, only phenylalanine is produced in
sufficient quantities, at normal aspartame intake levels, to cause
concern. In the normal healthy individual any phenylalanine
produced is harmless; however, it is recommended that aspartame
be avoided or its intake restricted by those persons with
phenylketonuria.
The WHO has set an acceptable daily intake for aspartame at up
to 40 mg/kg body-weight. Additionally, the acceptable daily
intake of diketopiperazine (an impurity found in aspartame) has
been set by the WHO at up to 7.5 mg/kg body-weight.
A number of adverse effects have been reported following the
consumption of aspartame, particularly in individuals who
drink large quantities (up to 8 liters per day in one case) of
aspartame-sweetened beverages. Reported adverse effects include:
headaches; grand mal seizure;memory loss;gastrointestinal
symptoms; and dermatological symptoms. However, scientifically
controlled peer-reviewed studies have consistently failed to
produce evidence of a causal effect between aspartame consumption
and adverse health events. Controlled and thorough studies
have confirmed aspartame’s safety and found no credible link
between consumption of aspartame at levels found in the human
diet and conditions related to the nervous system and behavior, nor
any other symptom or illness. Aspartame is well documented to be
nongenotoxic and there is no credible evidence that aspartame is
carcinogenic.
Although aspartame has been reported to cause hyperactivity
and behavioral problems in children, a double-blind controlled trial
of 48 preschool-age children fed diets containing a daily intake of
38 ± 13 mg/kg body-weight of aspartame for 3 weeks showed no
adverse effects attributable to aspartame, or dietary sucrose, on
children’s behavior or cognitive function.
Environmental Fate
Aspartame is nontoxic. However, individuals with the rare, genetic disease, phenylketonuria (PKU), cannot properly metabolize phenylalanine. Such individuals are detected by testing at birth and placed on special low-phenylalanine diets to control their blood phenylalanine concentrations. Thus, PKU individuals need to be aware that aspartame is a source of phenylalanine.
Metabolic pathway
The rate of aspartame degradation is faster in a phosphate buffer solution than in a citrate buffer solution at the same pH and buffer concentration. The primary mechanism by which aspartame degrades, the formation of diketo piperazine, involves the nucleophilic attack of carbonyl by the free amine, which requires proton transfer.
storage
Aspartame is stable in dry conditions. In the presence of moisture,
hydrolysis occurs to form the degradation products L -aspartyl-Lphenylalanine
and 3-benzyl-6-carboxymethyl-2,5-diketopiperazine
with a resulting loss of sweetness. A third-degradation product is
also known, β-L-aspartyl-L-phenylalanine methyl ester. For the
stability profile at 258℃ in aqueous buffers.
Stability in aqueous solutions has been enhanced by the addition
of cyclodextrins, and by the addition of polyethylene glycol 400
at pH 2. However, at pH 3.5–4.5 stability is not enhanced by the
replacement of water with organic solvents.
Aspartame degradation also occurs during prolonged heat
treatment; losses of aspartame may be minimized by using processes
that employ high temperatures for a short time followed by rapid
cooling.
The bulk material should be stored in a well-closed container, in
a cool, dry place.
Incompatibilities
Differential scanning calorimetry experiments with some directly compressible tablet excipients suggests that aspartame is incompatible with dibasic calcium phosphate and also with the lubricant magnesium stearate. Reactions between aspartame and sugar alcohols are also known.
Regulatory Status
Accepted for use as a food additive in Europe and in the USA. Included in the FDA Inactive Ingredients Database (oral powder for reconstitution, buccal patch, granules, syrups, and tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Aspartame Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | info@deshangchem.com | China | 662 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 | L18532138899@163.com | China | 939 | 58 |
| Aurora Industry Co., Ltd. | +86-13591747876 +86-13591747876 | alex1_auco@126.com | China | 277 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 | admin@hbdangtong.com | China | 1000 | 58 |
Related articles
- Aspartame: Chemical Structure, Digestion and Metabolism
- Aspartame, composed of aspartic acid and phenylalanine, undergoes digestion and metabolism with potential effects on neurotran....
- Jun 20,2024
- Recommended usage of Aspartame
- Based on the lack of toxicity in animal studies, a no-observed effect level of at least 4000 mg kg-1 body weight per day was e....
- Nov 16,2021
- The applications and Health effects of Aspartame
- Aspartame (APM) is an artificial non-saccharide sweetener used as a sugar substitute in some foods and beverages. It is a meth....
- Aug 16,2019
View Lastest Price from Aspartame manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | Aspartame
22839-47-0
|
US $0.00-0.00 / Kg/Bag | 1Kg/Bag | 0.99 | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-25 | Aspartame
22839-47-0
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd | |
 |
2024-11-25 | Aspartame
22839-47-0
|
US $2.00 / KG | 1KG | 99% | 200mt/year | Jinan Finer Chemical Co., Ltd |