Aspartame: Chemical Structure, Digestion and Metabolism
Dec 25,2024
General Description
Aspartame was first manufactured in 1965. In 1981, following a cursory assessment of its safety and toxicity, aspartame was approved by the U.S. Food and Drug Administration for use in foods. Today, with an annual production of 3000–5000 metric tons, aspartame is one of the world’s most widely used artificial sweeteners. It is an ingredient in over 5000 food and beverage products including cereals, chewing gum, yogurt, pharmaceuticals, and instant coffee. A particularly important use in the United States is manufacturing low-calorie beverages extensively consumed by children and pregnant women[1].
Chemical property

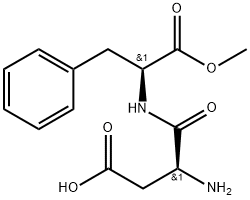
Aspartame is a dipeptide composed of two amino acids, l-aspartic acid and l-phenylalanine methyl ester. Chemically, aspartame is N-l-α-aspartyl-l-phenylalanine methyl ester or 3-amino-N-(α-carboxyphenethyl)succinamic acid N-methyl ester. It has a molecular formula of C14H18O5N2 and a molecular weight of 294.30. Aspartame is slightly soluble in water (about 1.0% at 25 °C), sparingly soluble in alcohol, and insoluble in fats and oils. The solubility in water is affected by temperature and pH. It increases as the pH is lowered and the temperature is increased.
It is 188 times sweeter than sugar, with the same calorie contents per weight unit. Aspartame is the methyl ester of a dipeptide composed of a hydrophilic and a hydrophobic amino acid residue, aspartic acid (Asp) and phenylalanine (Phe), respectively, giving it some unique qualities. Aspartame in purified solid form is a white crystalline powder that may be stored between 30 and 80 °C and is extremely stable under dry conditions. At room temperature, its aqueous solution has a half‐life of approximately 300 days and reaches the highest stability at a pH of 4.3, which is common for diet sodas. Or diet sodas. The peptide bonds are hydrolyzed in certain conditions, such as high temperatures or pH.
Digestion and Metabolism
Upon ingestion, aspartame is absorbed from the intestinal lumen and metabolized by its enzymes (esterase and peptidase) into three metabolites (amino acid isolates), phenylalanine (50%), aspartic acid (40%), and methanol (10%). In the liver, the phenylalanine hydroxylase enzyme processes phenylalanine into l-tyrosine. Then, the tyrosine hydroxylase enzyme converts l-tyrosine into l-dopa (l-3,4 dihydroxyphenylalanine). l-Dopa, in turn, is further transformed into catecholamines (dopamine, norepinephrine, and epinephrine) by the amino acid decarboxylase enzyme[2].
Aspartic acid (di-carboxyl amino acid) is metabolized in the liver by aspartate kinase enzyme into l-lysine and l-methionine. Multiple enzymes (species dependent) are involved in methanol metabolism into formaldehyde: alcohol dehydrogenase in primates and alcohol catalase in rodents. Formic acid, resulting from the oxidation of formaldehyde by formaldehyde dehydrogenase, is rapidly metabolized into carbon dioxide and water in rodents since rodents produce more folic acid (tetrahydrofolate) than primates. Humans are uniquely sensitive because of their low hepatic folate concentrations; excess formic acid may lead to metabolic acidosis and tissue injury.
References:
[1] SHUROOQ ASAAD ABDULAMEER SHAHER ?Bogdan A ?Dan Florin Mihailescu. Aspartame Safety as a Food Sweetener and Related Health Hazards.[J]. Nutrients, 2023, 15 16. DOI:10.3390/nu15163627.
[2] ALI E M T. Aspartame-Induced Neurodegeneration Versus Omega 3 Alleviation[J]. Omega Fatty Acids in Brain and Neurological Health, 1900, 33 2. DOI:10.1016/B978-0-12-815238-6.00023-7.
- Related articles
- Related Qustion
- Recommended usage of Aspartame Nov 16, 2021
Based on the lack of toxicity in animal studies, a no-observed effect level of at least 4000 mg kg-1 body weight per day was established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the European Food Safety Authority (EF
- The applications and Health effects of Aspartame Aug 16, 2019
Aspartame (APM) is an artificial non-saccharide sweetener used as a sugar substitute in some foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide. Aspartame was first made in 1965 and approved for use in food products by the U.S. Food and Drug Administration (FDA) in 1981.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDimethyl sulfone, versatile and stable, decomposes at high temps, vital in biology. Challenges in cryopreservation but diverse applications in skincare, supplements, and industry.....
Oct 25,2024Organic reagents