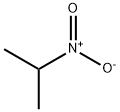
2-Nitropropane
- CAS No.
- 79-46-9
- Chemical Name:
- 2-Nitropropane
- Synonyms
- 2-NP;2-Nitropropane,96%;i-C3H7NO2;nipars-20;Nipar S-20;b-nitropropane;2-nitro-propan;2-NITROPROPANE;NITROISOPROPANE;ISONITROPROPANE
- CBNumber:
- CB4854412
- Molecular Formula:
- C3H7NO2
- Molecular Weight:
- 89.09
- MDL Number:
- MFCD00007397
- MOL File:
- 79-46-9.mol
- MSDS File:
- SDS
| Melting point | -93 °C |
|---|---|
| Boiling point | 120 °C(lit.) |
| Density | 0.992 g/mL at 25 °C(lit.) |
| vapor density | ~3 (vs air) |
| vapor pressure | ~13 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 99 °F |
| storage temp. | Flammables area |
| solubility | H2O: slightly soluble |
| form | Liquid |
| pka | pK1:7.675 (25°C) |
| color | Colorless to Almost colorless |
| Water Solubility | 1.7 g/100 mL (20 ºC) |
| Merck | 14,6628 |
| BRN | 1740684 |
| Henry's Law Constant | 8.92 at 20.00 °C, 15.3 at 30.00 °C, 24.4 at 40.00 °C, 36.9 at 50.00 °C (inert gas stripping, Bene? and Dohnal, 1999) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: IDLH 100 ppm; OSHA PEL: TWA 25 ppm (90 mg/m3); ACGIH TLV: TWA 10 ppm (adopted). |
| Dielectric constant | 25.5 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases, copper. |
| LogP | 1.35 at 20℃ |
| Indirect Additives used in Food Contact Substances | 2-NITROPROPANE |
| FDA 21 CFR | 175.105 |
| CAS DataBase Reference | 79-46-9(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | GKV234L2QH |
| Proposition 65 List | 2-Nitropropane |
| IARC | 2B (Vol. 29, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Propane, 2-nitro-(79-46-9) |
| EPA Substance Registry System | 2-Nitropropane (79-46-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H302-H331-H341-H350-H412 | |||||||||
| Precautionary statements | P202-P210-P273-P301+P312-P304+P340+P311-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 45-10-20/22-68-52/53 | |||||||||
| Safety Statements | 53-45-61-36/37 | |||||||||
| RIDADR | UN 2608 3/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TZ5250000 | |||||||||
| Autoignition Temperature | 802 °F | |||||||||
| HazardClass | 3.2 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29042000 | |||||||||
| Toxicity | Acute oral LD50 for rats 720 mg/kg (quoted, RTECS, 1985). | |||||||||
| IDLA | 100 ppm | |||||||||
| NFPA 704 |
|
2-Nitropropane price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 130265 | 2-Nitropropane ≥96% | 79-46-9 | 5ml | $44.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 130265 | 2-Nitropropane ≥96% | 79-46-9 | 100ml | $54.2 | 2024-03-01 | Buy |
| TCI Chemical | N0249 | 2-Nitropropane >95.0%(GC) | 79-46-9 | 25g | $118 | 2024-03-01 | Buy |
| TCI Chemical | N0249 | 2-Nitropropane >95.0%(GC) | 79-46-9 | 100g | $161 | 2024-03-01 | Buy |
| Sigma-Aldrich | 130265 | 2-Nitropropane ≥96% | 79-46-9 | 500ml | $213 | 2024-03-01 | Buy |
2-Nitropropane Chemical Properties,Uses,Production
Description
2-Nitropropane, also known as dimethylnitromethane or isonitropropane, is a colourless, oily liquid with a mild and sweet odour. This flammable compound is soluble in water and various organic solvents, including chloroform. Its vapors can form an explosive mixture when combined with air. 2-Nitropropane serves as a co-solvent in paint formulations, enhancing pigment wetting, optimizing flow properties, and facilitating electrostatic processing. Additionally, it contributes to a shorter paint drying time.
Chemical Properties
2-Nitropropane, also known as 2-NP, is an aliphatic nitro compound that appears as a colourless, flammable liquid with a mild fruity odour. It can dissolve in water (1.7 mL/100 mL at 20C) and is miscible with organic solvents.
Like other nitroalkanes, 2-nitropropane exists in equilibrium with 2-propane nitronic acid (HSDB 1988). A 0.01 M aqueous solution of 2-NP has a pH of 6.2. When reacted with inorganic bases and amines, 2-nitropropane forms salts that are flammable when dry. It is sensitive to detonation when combined with oxidizers, and a mixture of 2-nitropropane and ammonium nitrate serves as a commercial explosive.
Physical properties
Colorless, oily liquid with a mild, fruity odor. 2-Nitropropane was detected in two studies at concentrations of 3.1 and 5.2 ppmv (Crawford et al., 1984).
Uses
2-Nitropropane is primarily used as a solvent for organic compounds and coatings; with vinyl resins, epoxy paints, nitrocellulose, and chlorinated rubber; in printing inks, adhesives, and printing as flexographic inks; maintenance with traffic markings on roads and highways; shipbuilding; and general maintenance. It also has limited use as a paint and varnish remover. 2-Nitropropane is also used as a solvent in food processing industries for fractionation of a partially saturated vegetable oil.
Preparation
The synthesis of 2-nitropropane can be accomplished directly by nitration of 2-halopropanes with sodium nitrite. With 2-iodopropane, the reaction is carried out in dry DMF in the presence of urea. For the slower reacting 2-bromopropane, a longer reaction time and the presence of phloroglucinol as a nitrite ester scavenger is required.
Like 1-nitropropane, 2-nitropropane is also produced by vapor-phase nitration of propane with nitric acid at elevated temperature and pressure (Baker and Bollmeier 1978).
Definition
ChEBI: 2-nitropropane is a secondary nitroalkane that is propane in which a hydrogen at position 2 has been replaced by a nitro group. Mainly used as a solvent (b.p. 120℃). It has a role as a hepatotoxic agent, a carcinogenic agent, a polar aprotic solvent and a xenobiotic.
General Description
2-Nitropropane is a clear, colorless liquid with a pleasant odor that is soluble in many organic solvents, including chloroform. Its vapors may form an explosive mixture with air.
Air & Water Reactions
Highly flammable.
Reactivity Profile
2-Nitropropane is sensitive to heat. Can react with amines/heavy metal oxides, strong acids, strong alkalis, and chlorosulfonic acid. . The heat of adsorption of 2-Nitropropane on carbon, such as that found in cartridge respirators, is extremely high. Metal oxide catalysts, such as copper oxide or manganese oxide, can initiate ignition, therefore carbon respirators should not be used in environments that have a high vapor concentration of 2-Nitropropane.
Health Hazard
2-Nitropropane is a lung irritant. The acute effects of exposure to 2-nitropropane at 20-45 p.p.m. on workmen were anorexia, nausea, vomiting, diarrhea and severe occipital headache. Liver effects have been observed in animals chronically exposed to 2-nitropropane by inhalation.
Carcinogenicity
2-Nitropropane was classified by the International Agency for Research on Cancer (IARC) as Group 2B, possibly carcinogenic to humans, based on animal studies linking exposure to liver cancer in rats.The carcinogenicity of 2-nitropropane in humans was not evaluated due to the lack of adequate epidemiological data.
Environmental Fate
Photolytic. Anticipated products from the reaction of 2-nitropropane with ozone or OH radicals in the atmosphere are formaldehyde and acetaldehyde (Cupitt, 1980).
Shipping
UN2608 Nitropropanes, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Purify it as for nitromethane. [Beilstein 1 IV 230.]
Toxicity evaluation
DNA damage can cause 2-NP metabolites such as N-isopropyl hydroxylamine (IPHA) and hydroxylamine-O-sulfonic (HAS) acid by a reactive oxygen generating process that can be inhibited by free hydroxyl radical scavengers, catalase, and deferoxamine mesylate, an iron chelating agent. IPHA causes DNA damage at thymine and HAS most frequently induces DNA damage at 5'-TG-3', 5'-GG-3', and 5' -GGG-3' sequences. Formation of 8-oxodesoxyguanine by IPHA or HAS increased in the presence of metal ions.DNA damage caused by 2-NP metabolites plays an important role in mutagenicity and carcinogenicity of 2-NP. The liver damage induced by 2-NP is related to oxidative damage and reduction in catalase (CAT) activity.
Incompatibilities
1-Nitropropane, a nitroparaffin compound, forms explosive mixture with air. Contact with heavy metal oxides may cause decomposition. Mixtures with hydrocarbons are extremely flammable. Attacks some plastics, rubber and coatings. May explode on heating. Violent reaction with strong bases; strong acids and metal oxides. Shock-sensitive compounds are formed with acids, amines, inorganic bases and heavy metal oxides. Incompatible with strong oxidizers, combustible materials. 2-Nitropropane reacts with activated carbon causing decomposition. This reaction may occur in activated carbon respirator filters.
Waste Disposal
Incineration: large quantities of material may require nitrogen oxide removal by catalytic or scrubbing processes. Dilute with pure kerosene and burn with care as it is potentially explosive. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
2-Nitropropane Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Risun Science and Technology Limited | +86-13311342578 +86-19801193650 | xykj@risun.com | China | 13 | 58 |
| Chemsigma International Co., Ltd. | +86-18136843612 +86-19951791336 | info@chemsigma.com | China | 1350 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21639 | 55 |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | stella@zetchem.com | China | 2136 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 8497 | 58 |
| Shanghai Worldyang Chemical Co.,Ltd. | +86-021-56795779 +8613651600618 | sales@worldyachem.com | China | 1073 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
Related articles
- Preparation and Uses of 2-Nitropropane
- 2-Nitropropane is used primarily as a solvent. Severe liver damage, as well as some kidney damage, has been observed in worke....
- Jan 19,2022
View Lastest Price from 2-Nitropropane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-11 | 2-Nitropropane
79-46-9
|
US $100.00-50.00 / KG | 1KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-08-05 | 2-Nitropropane
79-46-9
|
US $90.00-50.00 / kg | 1kg | 99% | 5MT/month | Shanghai Bojing Chemical Co.,Ltd. | |
 |
2023-12-18 | 2-Nitropropane
79-46-9
|
US $0.00-0.00 / KG | 1KG | 99%(GC) | 20 tons | Beijing Risun Science and Technology Limited |
-

- 2-Nitropropane
79-46-9
- US $100.00-50.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- 2-Nitropropane
79-46-9
- US $90.00-50.00 / kg
- 99%
- Shanghai Bojing Chemical Co.,Ltd.
-

- 2-Nitropropane
79-46-9
- US $0.00-0.00 / KG
- 99%(GC)
- Beijing Risun Science and Technology Limited