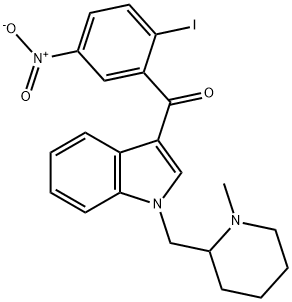
(R,S)-AM1241
- CAS No.
- 444912-48-5
- Chemical Name:
- (R,S)-AM1241
- Synonyms
- Methanone, (2-iodo-5-nitrophenyl)[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]-;(R,S)-AM1241;AM1241 (AM-1241;(R,S)-AM1241 USP/EP/BP;(R,S)-3-(2-Iodo-5-nitrobenzoyl)-1-(1-methyl-2-piperidinylmethyl)-1H-indole;(3-iodo-5-nitrophenyl)-[1-[(1-methylpiperidin-2-yl)methyl]indol-3-yl]methanone;(2-iodo-5-nitrophenyl)-[1-[(1-methylpiperidin-2-yl)methyl]indol-3-yl]methanone;(2-Iodo-5-nitrophenyl)[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]methanone;(2-Iodo-5-nitrophenyl)(1-((1-methylpiperidin-2-yl)methyl)-1H-Indol-3-yl)methanone
- CBNumber:
- CB4972473
- Molecular Formula:
- C22H22IN3O3
- Molecular Weight:
- 503.33
- MDL Number:
- MFCD09970778
- MOL File:
- 444912-48-5.mol
| Boiling point | 630.7±55.0 °C(Predicted) |
|---|---|
| Density | 1.60±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO: ~18mg/mL at 60°C |
| pka | 9.73±0.10(Predicted) |
| form | solid |
| color | yellow |
| FDA UNII | DLM851L3RD |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H319-H334-H335 | |||||||||
| Precautionary statements | P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 36/37/38-42/43 | |||||||||
| Safety Statements | 22-26-36/37-45 | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
(R,S)-AM1241 price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A6478 | (R,S)-AM1241 ≥98% (HPLC), solid | 444912-48-5 | 5mg | $264 | 2024-03-01 | Buy |
| Sigma-Aldrich | A6478 | (R,S)-AM1241 ≥98% (HPLC), solid | 444912-48-5 | 25mg | $380.8 | 2024-03-01 | Buy |
| Cayman Chemical | 10010118 | AM1241 ≥97% | 444912-48-5 | 1mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 10010118 | AM1241 ≥97% | 444912-48-5 | 5mg | $78 | 2024-03-01 | Buy |
| Cayman Chemical | 10010118 | AM1241 ≥97% | 444912-48-5 | 10mg | $139 | 2024-03-01 | Buy |
(R,S)-AM1241 Chemical Properties,Uses,Production
Description
AM1241 is a cannabinoid (CB) receptor agonist that is selective for CB2 over CB1 with Ki values of 7.1 and 580 nM for human recombinant receptors transfected into HEK and CHO cells, respectively, in a radioligand binding assay. It is considered a protean agonist as it has neutral antagonist and partial agonist activity, depending on the assay utilized. It is also acts in a species-dependent manner in vitro, acting as an agonist at human CB2 receptors (EC50 = 190 nM) but an inverse agonist at rat and mouse CB2 receptors (EC50s = 216 and 463 nM, respectively). AM1241 produces antinociception to thermal stimuli in rat hindpaw. The antinociceptive actions of AM1241 were blocked by the CB2 receptor-selective antagonist AM630 but not by the CB1 receptor-selective antagonist AM251 . AM1241 is neuroprotective, preventing HIV-1 glycoprotein Gp120-induced apoptosis in primary human and murine neural progenitor cells and increasing cell survival and differentiation. It increases hippocampal neurogenesis and decreases astro- and gliogenesis in GFAP/Gp120 transgenic mice when administered at a dose of 10 mg/kg daily for ten days. AM1241 also delays motor impairment in a murine model of amytrophic lateral sclerosis (ALS).
Uses
(R,S)-AM1241 has been used as a cannabinoid CB2 agonist:
- to study its inhibitory effect on bone cancer-induced pain and bone loss
- to study the effect of its interaction with 17βestradiol on proliferation activity in primary human osteoblasts
- to evaluate the sites of CB2 mediated antinociception in vivo.
Uses
(2-Iodo-5-nitrophenyl)[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]methanone is a compound from the aminkalkylindole family which exerts potent and selective agonist activity for the cannabinoid receptor CB2.
Biochem/physiol Actions
AM1241 acts as an antinociceptive agent in several animal pain models. It has a potential to delay disease progression in amyotrophic lateral sclerosis (ALS) mouse model. Intrathecal, intravenous or intraperitoneal administration of AM1241 reduces hyperalgesia and allodynia in neuropathic rats.
target
CB2
(R,S)-AM1241 Preparation Products And Raw materials
Raw materials
Preparation Products
(R,S)-AM1241 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32956 | 60 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22787 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38631 | 58 | |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131957 | 58 |
| China DongFan Chemical Co.,LTD | 86-0571-85151182 | China | 5691 | 66 |