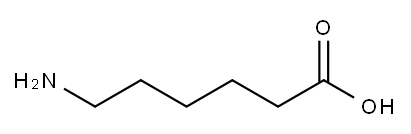
6-Aminocaproic acid
- CAS No.
- 60-32-2
- Chemical Name:
- 6-Aminocaproic acid
- Synonyms
- 6-AMINOHEXANOIC ACID;ACS;Aminocaproic;6-aminohexanoate;EACA;Amicar;H-ACP-OH;Hepin;177 J.D;aminocaproic acid,EACA
- CBNumber:
- CB5223888
- Molecular Formula:
- C6H13NO2
- Molecular Weight:
- 131.17
- MDL Number:
- MFCD00008238
- MOL File:
- 60-32-2.mol
- MSDS File:
- SDS
| Melting point | 207-209 °C (dec.) (lit.) |
|---|---|
| Boiling point | 242.49°C (rough estimate) |
| Density | 1.042 g/cm3 |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | 1.4870 (estimate) |
| Flash point | 207-209°C |
| storage temp. | Store below +30°C. |
| solubility | H2O: 50 mg/mL |
| form | powder |
| pka | 4.373(at 25℃) |
| color | white |
| Odor | Odorless |
| PH | 7.0-7.5 (50g/l, H2O, 20℃) |
| Water Solubility | SOLUBLE |
| Merck | 14,432 |
| BRN | 906872 |
| InChIKey | SLXKOJJOQWFEFD-UHFFFAOYSA-N |
| LogP | 0.027 (est) |
| CAS DataBase Reference | 60-32-2(CAS DataBase Reference) |
| EWG's Food Scores | 1-3 |
| FDA UNII | U6F3787206 |
| NCI Drug Dictionary | Amicar |
| ATC code | B02AA01 |
| NIST Chemistry Reference | Hexanoic acid, 6-amino-(60-32-2) |
| EPA Substance Registry System | Hexanoic acid, 6-amino- (60-32-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H320-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a-P264-P305+P351+P338+P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | MO6300000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29224995 | |||||||||
| Toxicity | LD50 in rats (g/kg): 7.0 i.p.; ~3.3 i.v. (Hallesy) | |||||||||
| NFPA 704 |
|
6-Aminocaproic acid price More Price(71)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.00145 | 6-Aminohexanoic acid for synthesis | 60-32-2 | 5G | $33.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00145 | 6-Aminohexanoic acid for synthesis | 60-32-2 | 250g | $77.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00145 | 6-Aminohexanoic acid for synthesis | 60-32-2 | 1kg | $280 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00145 | 6-Aminohexanoic acid for synthesis | 60-32-2 | 5kg | $966 | 2024-03-01 | Buy |
| Sigma-Aldrich | 07260 | 6-Aminohexanoic acid ≥98.5% (NT) | 60-32-2 | 100g | $146 | 2024-03-01 | Buy |
6-Aminocaproic acid Chemical Properties,Uses,Production
Description
6-aminocaproic acid (Brand name: Amicar) is a kind of synthetic derivative of lysine. Since it is a analogue of amino acid lysine, it can act as the inhibitor for enzymes that need to bind to that particular lysine residue, e.g. the proteolytic enzyme such as plasmin, which is responsible for fibrinolysis. Therefore, it has anti-fibrinolytic activity. It also competitively inhibits activation of plasminogen, thereby reducing conversion of plasminogen to plasmin. Based on this property, it can be used for the treatment of acute bleeding due to elevated fibrinolytic activity in many clinical situations. It can also indicated by FDA for the prevention of recurrent hemorrhage in patients of traumatic hyphema. It may also act as a prophylactic against the vascular disease due to its inhibitory effect on the formation of lipoprotein which is the risk factor of vascular disease.
References
https://pubchem.ncbi.nlm.nih.gov/compound/6-aminohexanoic_acid#section=Pharmacology-and-Biochemistry
https://en.wikipedia.org/wiki/Aminocaproic_acid
Chemical Properties
white crystalline powder. Leaf crystals were obtained from ether. Odorless, bitter taste. Melting point 202 ~ 207 ℃ (decomposition). Soluble in water, slightly soluble in methanol, insoluble in ethanol, ether and chloroform.
Originator
Epsilon,Roche,W. Germany,1962
Uses
EACA is directly soluble in water at 25 mg/ml. As an inhibitor of plasmin it is has been utilized in the clotting buffer for fibrinogen assays. This buffer is 10 mM potassium and sodium phosphate, pH 6.4, with 0.20 g CaCl2, 5 g 6-Aminohexanoic acid, 1 g sodium azide, and 9 g NaCl in 1 liter. The buffer is stable indefinitely at room temperature.
Uses
6-Aminohexanoic Acid is a reagent commonly used for the extraction of aldehydes from reaction mixtures. 6-Aminohexanoic Acid has also been shown to improve solubilization of membrane proteins in elect rophoresis. Studies suggest that 6-Aminohexanoic Acid inhibits the activation of the first component of the complement system.
Definition
ChEBI: 6-aminohexanoic acid is an epsilon-amino acid comprising hexanoic acid carrying an amino substituent at position C-6. Used to control postoperative bleeding, and to treat overdose effects of the thrombolytic agents streptokinase and tissue plasminogen activator. It has a role as an antifibrinolytic drug, a hematologic agent and a metabolite. It is an epsilon-amino acid and an omega-amino fatty acid. It derives from a hexanoic acid. It is a conjugate acid of a 6-aminohexanoate. It is a tautomer of a 6-aminohexanoic acid zwitterion.
Preparation
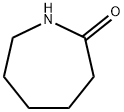
6-aminocaproic acid is obtained by hydrolysis of 6-(N-benzoylamino)capronitrile in the presence of hydrochloric acid, or by hydrolysis of caprolactam in the presence of hydrochloric acid, and then treated with ammonium hydroxide.
Application
6-Aminohexanoic acid was used as a biochemical reagent. It is also used as a hemostatic agent. 6-aminocaproic acid has a significant effect on some severe bleeding caused by increased fibrinolytic activity. It is suitable for oozing or local bleeding during various surgical operations. 6-aminocaproic acid is used for hemoptysis, gastrointestinal bleeding and bleeding disorders in obstetrics and gynecology.
6-Aminocaproic acid is an anti-fibrinolytic drug with a similar chemical structure to lysine. It can qualitatively inhibit the binding of plasminogen to fibrin and prevent its activation, thereby inhibiting fibrinolysis and achieving hemostasis. Aminocaproic acid is a monoaminocarboxylic acid, which can inhibit the conversion of plasminogen into plasmin and its binding to fibrin. For severe bleeding caused by hyperfibrinolysis caused by increased activation of plasminogen, can have therapeutic effect.
Manufacturing Process
5 kg of caprolactam were heated with 40 liters of water in a pressure vessel at 250°C for a period of four hours. These quantities of reactants correspond to a water:lactam molecular ratio of 50:1. After cooling, the small quantity of the nonsoluble substance that is formed is filtered off, and the filtrate is evaporated as far as possible. The resulting concentrate is mixed with three times its volume of strong alcohol, thereby causing the desired product, epsilon-aminocaproic acid (6-aminohexanoic acid), to crystallize out. After separating the crystalline product thus obtained, a further quantity of epsilonaminocaproic acid can be obtained from the mother liquid if desired.
brand name
Amicar (Xanodyne).
Therapeutic Function
Antifibrinolytic
General Description
6-Aminocaproic acid is a synthetic inhibitor of fibrinolysis and is utilized for the control of excessive bleeding in patients with amegakaryocytic thrombocytopenia. It also is a protease inhibitor that displays anticancer activity but is limited by cytotoxicity.
Biochem/physiol Actions
Lysine analog. Promotes rapid dissociation of plasmin, thereby inhibiting the activation of plasminogen and subsequent fibrinolysis. Reported to inhibit plasminogen binding to activated platelets. An early report indicated that it inhibits the activation of the first component of the complement system. Binds and inactivates Carboxypeptidase B.
Mechanism of action
Because binding of plasminogen or plasmin to fibrinogen or fibrin is mediated by lysine groups that are part of the structures of fibrin and fibrinogen, aminocaproic acid, which is a structural analog of lysine that only differs in that it has one less amino group, acts as a competitive inhibitor for binding of plasmin(ogen) to fibrin. Aminocaproic acid shifts the homeostatic balance on the side of coagulation, thus restoring fibrinolytic mechanism activity. Aminocaproic acid, which is not a procoagulant, such as those used during surgical intervention and various pathological conditions, is accompanied by an elevation in fibrinolytic activity of blood and tissue. It is used to stop bleeding.
Safety Profile
Moderately toxic by intravenous route. Human systemic effects by ingestion: changes in tubules (includmg acute renal failure, acute tubular necrosis), hematuria, and increased body temperature. Experimental reproductive effects. An eye irritant. When heated to decomposition it emits toxic fumes such as NOx,.
Synthesis
Aminocaproic acid (24.4.1) is synthesized by hydrolyzing |? -caprolactam at high temperature.
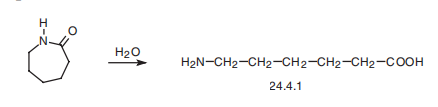
Veterinary Drugs and Treatments
Aminocaproic acid has been used as a treatment to degenerative myelopathy (seen primarily in German shepherds), but no controlled studies documenting its efficacy were located. There is interest in evaluating aminocaproic acid for adjunctive treatment of thrombocytopenia in dogs, but efficacy and safety for this purpose remains to be investigated. In humans, it is primarily used for treating hyperfibrinolysis-induced hemorrhage.
6-Aminocaproic acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Fortuna Chemical Co.,Ltd | +8618007136271 | hk@fortunachem.com | China | 5998 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29883 | 58 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 | info@royal-chem.com | China | 114 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18154 | 58 |
| Sichuan HongRi Pharma-Tech Co.,Ltd | +86-028-64841719 +8617390183901 | daisy@enlaibio.com | China | 1106 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
Related articles
- 6-Aminocaproic Acid: Medical Applications and Spectrofluorimetric Determination
- 6-Aminocaproic acid enhances cancer therapy by improving ICG stability and efficacy, while its spectrofluorimetric determinati....
- Jun 17,2024
- The preparation of 6-aminocaproic
- 6-Aminocaproic acid is an organic substance with the molecular formula of C6H13O2N, NH2(CH2)5COOH and a white crystalline powd....
- Apr 13,2022
View Lastest Price from 6-Aminocaproic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | 6-Aminocaproic acid
60-32-2
|
US $0.00-0.00 / kg | 1kg | 99% | 1T+ | Sichuan HongRi Pharma-Tech Co.,Ltd | |
 |
2024-11-22 | 6-Aminocaproic acid
60-32-2
|
US $0.00 / KG | 25KG | 99 | 2mt | Yancheng Green Chemicals Co.,Ltd | |
 |
2024-11-20 | 6-Aminocaproic acid
60-32-2
|
US $0.00-0.00 / kg | 1kg | 99% | 500kg | Wuhan Fortuna Chemical Co.,Ltd |
-

- 6-Aminocaproic acid
60-32-2
- US $0.00-0.00 / kg
- 99%
- Sichuan HongRi Pharma-Tech Co.,Ltd
-

- 6-Aminocaproic acid
60-32-2
- US $0.00 / KG
- 99
- Yancheng Green Chemicals Co.,Ltd
-

- 6-Aminocaproic acid
60-32-2
- US $0.00-0.00 / kg
- 99%
- Wuhan Fortuna Chemical Co.,Ltd
60-32-2(6-Aminocaproic acid)Related Search:
1of4